| In vitro: |
| Yao Xue Xue Bao. 2008 Nov;43(11):1119-22. | | [2-Arylbenzofuran derivatives from Morus wittiorum].[Pubmed: 19239031] | The investigation on the stem bark of Morus wittiorum was carried out to find its chemical constituents possessing anti-oxidative activity.
METHODS AND RESULTS:
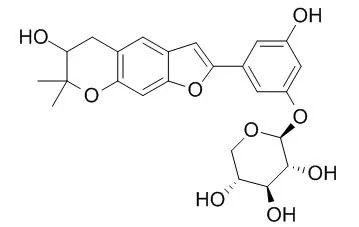
The isolation and purification were performed by various chromatographies such as silica gel, Sephadex LH-20, RP-C18 column chromatography and so on. Based on the spectral analysis such as NMR, MS, etc., seven 2-arylbenzofuran derivatives were identified as wittifuran D (1), wittifuran E (2), moracin C (3), moracin M (4), moracin P (5), 2-(3,5-dihydroxyphenyl)-5,6-dihydroxybenzofuran (6) and Mulberroside C (7). Compounds 1-7 were isolated from this plant for the first time. Among them, 1 and 2 were new compounds. Compounds 3-7 were used to assay antioxidant activity, the inhibitory ratios of compounds 3, 4, 6, at a concentration of 1 x 10(-5) mol x L(-1), to malondialdehyde (MDA) produced during microsomal lipid peroxidation induced by ferrous-cysteine were 73%, 69% and 89% respectively. | | Phytochemistry. 2003 Apr;62(8):1235-8. | | Antiviral flavonoids from the root bark of Morus alba L.[Pubmed: 12648543] |
METHODS AND RESULTS:
A prenylated flavonoid, moralbanone, along with seven known compounds kuwanon S, Mulberroside C, cyclomorusin, eudraflavone B hydroperoxide, oxydihydromorusin, leachianone G and alpha-acetyl-amyrin were isolated from the root bark of Morus alba L. Leachianone G showed potent antiviral activity (IC(50) = 1.6 microg/ml), whereas Mulberroside C showed weak activity (IC(50) = 75.4 microg/ml) against herpes simplex type 1 virus (HSV-1).
CONCLUSIONS:
Their structures were elucidated by spectroscopic methods. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)