| Cell Research: |
| Chinese Traditional & Herbal Drugs, 2016, 117(6):238-238. | | Chemical constituents from fruiting bodies of Amauroderma rude[Reference: WebLink] | To study the chemical constituents from the fruiting bodies in Amauroderma rude of family Ganodermataceae.
METHODS AND RESULTS:
The constituents were separated by column chromatography and their structures were elucidated by spectral data analyses. The cytotoxicities of compounds were evaluated using MTT methods. Twelve compounds were isolated and identified from the fruiting bodies of A. rude and were identified as diptoindonesin D(1), 6-Deoxyjacareubin(2), jacareubin(3), 1H-indole-3-carboxylic acid(4), methyl 3,4-dihydroxybenzoate(5), 3,4-dihydroxyphenylethanol(6), 3β-hydroxy-7,22E-dien-ergosta(7), 3β,7α-dihydroxy-8,22E-5α,6α-epoxyergosta(8), 3β-hydroxy-7α-methoxy-8(14),22E-dien-5α,6α-epoxyergosta(9), ergosterol 5α,8α-peroxide(10), 3β-5β-8β-trihydroxy-6,22E-ergosta(11), and 3β,5α-6β-trihydroxy-7,22E-dien-ergosta(12). Compounds 2 and 3 exhibited the cytotoxic activities against HL-60, SMMC-7721, A-549, MCF-7, and SW-480 cell lines and compound 9 showed the cytotoxic activities against HL-60, MCF-7, and SW-480 cell lines.
CONCLUSIONS:
All the compounds are obtained from this fungus for the first time. Compounds 2, 3, and 9 show the definite cytotoxicities against five cell lines. |
|
| Structure Identification: |
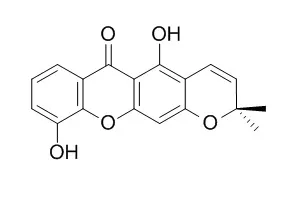
| Phytochemistry. 1994 Aug;36(6):1381-5. | | An antifungal gamma-pyrone and xanthones with monoamine oxidase inhibitory activity from Hypericum brasiliense.[Pubmed: 7765428] |
METHODS AND RESULTS:
A new gamma-pyrone (hyperbrasilone), three known xanthones (1,5-dihydroxyxanthone, 5-hydroxy-1-methoxyxanthone and 6-Deoxyjacareubin) and betulinic acid have been isolated from a dichloromethane extract of stems and roots of Hypericum brasiliense. Their structures were established by spectroscopic methods (UV, EI-MS, 1H and 13C NMR) and that of the gamma-pyrone was confirmed by X-ray crystallography.
CONCLUSIONS:
Hyperbrasilone and the xanthones were all antifungal against Cladosporium cucumerinum, while the three xanthones showed differing degrees of inhibition of monoamine oxidase A and B. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)