| Structure Identification: |
| Planta Medica, 2007, 73(9). | | Biotransformation of digitoxigenin – a plant secondary metabolite – by the fungus Cochliobolus lunatus.[Reference: WebLink] | Cardiac glycosides are plant secondary metabolites used to treat congestive heart failure. They also exhibit a wide spectrum of biological activities, including anti-carcinogenic, acaricidal, antifilarial, molluscicidal and antibacterial properties. Biotransformation of cardenolides has been investigated either as a strategy to obtain new derivatives or to convert the A-type cardenolides into the corresponding C-type compounds, which have clinical relevance [1].
METHODS AND RESULTS:
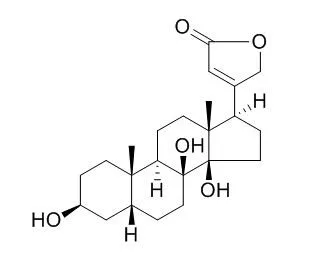
The fungus Cochliobolus lunatus and its conidial anamorphous form Curvularia lanata are known for their capacity of hydroxylating Δ4–5 steroids at position 11β, 14α and 7α [2]. The biotransformation of digitoxigenin 1, the aglycone of digitoxin, by C. lunatus was investigated. The biotransformation reaction was carried out in a 4-day process, resulting in the isolation of four products. Their structures were elucidated by 1D and 2D NMR data as 1β-hydroxydigitoxigenin 2, 7β-hydroxydigitoxigenin 3, 8β-hydroxydigitoxigenin(8-Hydroxydigitoxigenin) 4 and digitoxigenone 5 (Fig.1). The observed hydroxylation sites are distinct from those previously described for Δ4–5 steroids in reactions with C. lunatus. The production of 4 by a biotransformation reaction is clear evidence that C. lunatus hydroxylases involved in the reaction are not affected by the steric hindrance of the 14β-OH group [3].
CONCLUSIONS:
Therefore, it is feasible to obtain a product with two vicinal hydroxyls at 8β and 14β-positions. Hydroxylation of 1 at 1β-position is of special interest, since some glycosides of 1β-hydroxydigitoxigenin have been reported to exhibit potent in vitro activity against ovarian adenocarcinoma and lung carcinoma [4]. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)