| In vitro: |
| Phytother Res. 2009 Jul;23(7):938-42. | | Cytoprotective effect of lignans from Forsythia suspensa against peroxynitrite-induced LLC-PK1 cell damage.[Pubmed: 19367664] | There is mounting evidence that peroxynitrite (ONOO(-)) is closely related to the pathogenesis of various diseases.
METHODS AND RESULTS:
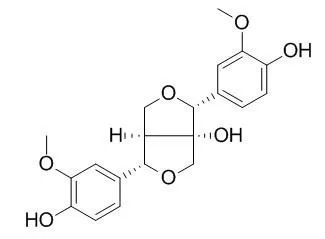
As a pharmacological strategy aimed at preventing ONOO(-)-mediated toxicity, the protective activity of Forsythia suspensa (Thunb.) Vahl (Oleaceae) against ONOO(-)-induced cellular damage was investigated and its active components identified. After bioactivity-guided fractionation of its methylene chloride fraction, two tetrahydrofurofuran lignans were isolated, namely phillygenin and 8-Hydroxypinoresinol. The protective effects of these lignans against ONOO(-)-induced cell death were evaluated using renal epithelial cell LLC-PK1. Phillygenin and 8-Hydroxypinoresinol significantly reduced the cell injury by 3-morpholinosydnonimine (SIN-1), a ONOO(-) generator. The hydroxy substituents on the phenyl moieties may contribute to the antioxidant activities of these lignans.
CONCLUSIONS:
These results suggest that phillygenin and 8-Hydroxypinoresinol may be useful for the therapeutic or preventive applications in treating ONOO(-)-related diseases. | | Arch Pharm Res. 2011 Dec;34(12):2029-35. | | Lignans from the flowers of Osmanthus fragrans var. aurantiacus and their inhibition effect on NO production.[Pubmed: 22210027] |
METHODS AND RESULTS:
A new lignan, (7R,7'R,8R,8'R)-8-Hydroxypinoresinol 8-O-β-D-glucopyranoside 4'-methyl ether (7), was isolated from the flowers of Osmanthus fragrans var. aurantiacus along with six known lignans: (+)-phillygenin (1), phillyrin (2), (-)-phillygenin (3), (-)-epipinoresinol-β-D-glucoside (4), taxiresinol (5), and (-)-olivil (6). The structure of the new compound was elucidated on the basis of 1D- and 2D-NMR spectroscopic analysis and specific rotation data. The compounds isolated from the flowers of O. fragrans var. aurantiacus were evaluated for inhibitory activities on nitric oxide production in lipopolysaccharide-stimulated macrophage RAW 264.7 cells. (+)-Phillygenin (1), phillyrin (2), and (-)-phillygenin (3) exerted the strongest inhibitory activities on NO production with IC(50) values of 25.5, 18.9, and 25.5 μM, respectively.
CONCLUSIONS:
These compounds may prove beneficial in the development of natural agents for prevention and treatment of inflammatory diseases. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)