| In vitro: |
| Arch Pharm Res. 2010 Mar;33(3):381-5. | | Naphthoquinones from Catalpa ovata and their inhibitory effects on the production of nitric oxide.[Pubmed: 20361302] |
METHODS AND RESULTS:
Bioassay-guided fractionation of a CH2Cl2-soluble fraction of the stems of Catalpa ovata led to isolation of a new naphthoquinone, 4-hydroxy-2-(2-methoxy-3-hydroxy-3-methyl-but-1-enyl)-4-hydro-1H-naphthalen-1-one (10), together with nine known compounds, catalponol (1), catalponone (2), catalpalactone (3), alpha-lapachone (4), 9-hydroxy-alpha-lapachone (5), 4,9-dihydroxy-alpha-lapachone (6), 9-Methoxy-alpha-lapachone (7), 4-oxo-alpha-lapachone (8), and 9-methoxy-4-oxo-alpha-lapachone (9). The structures were elucidated on the basis of spectroscopic analyses. The inhibitory effects of these isolates on lipopolysaccharide-induced NO synthesis in RAW 264.7 cells were evaluated.
CONCLUSIONS:
Among them, catapalactone (3), 9-hydroxy-alpha-lapachone (5) and 4,9-dihydroxy-alpha-lapachone (6) exhibited potent inhibitory effects, with IC(50) values of 9.80, 4.64 and 2.73 microM, respectively. | | J Nat Prod. 1998 May;61(5):629-32. | | Antitumor-promoting naphthoquinones from Catalpa ovata.[Pubmed: 9599262 ] |
METHODS AND RESULTS:
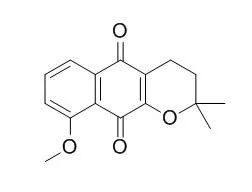
Bioassay-directed fractionation of an extract of the stem-bark of Catalpa ovata led to the isolation of three new naphthoquinones: 8-methoxydehydroiso-alpha-lapachone (1), 9-methoxy-4-oxo-alpha-lapachone (2), and (4S,4aR,10R,10aR)-4, 10-dihydroxy-2,2-dimethyl-2,3,4,4alpha,10, 10alpha-hexahydrobenzo[g]chromen-5-one (3), which is a 1,4-reductive form of 6. The known compounds 3-hydroxydehydroiso-alpha-lapachone (4), 4,9-dihydroxy-alpha-lapachone (5), 4-hydroxy-alpha-lapachone (6), and 9-Methoxy-alpha-lapachone (7), and catalpalactone (8) were also isolated. Their structures were elucidated by spectral methods.
CONCLUSIONS:
These compounds all exhibited significant inhibitory activity against 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced Epstein-Barr virus early antigen (EBV-EA) activation in Raji cells. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)