| Description: |
Demethoxycurcumin is a potential additive natural product in combination with chemotherapeutic agents in drug-resistant cancers, which has anti-acanthamoebic, anti-proliferative, antimetastatic, anti-inflammatory, antioxidant activities. It inhibited P-glycoprotein-mediated ATP hydrolysis under concentrations of <1 μM and efficiently inhibited 200 μM verapamil-stimulated ATPase activity. |
| In vitro: |
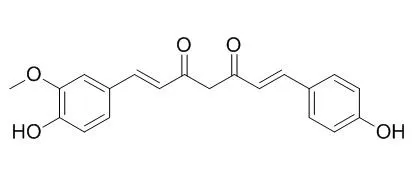
| J Agric Food Chem. 2013 Jul 3;61(26):6366-75. | | Demethoxycurcumin inhibits energy metabolic and oncogenic signaling pathways through AMPK activation in triple-negative breast cancer cells.[Pubmed: 23777448] | Demethoxycurcumin (DMC), curcumin (Cur), and bisDemethoxycurcumin (BDMC) are major forms of curcuminoids found in the rhizomes of turmeric.
METHODS AND RESULTS:
This study examined the effects of three curcuminoid analogues on breast cancer cells. The results revealed that DMC demonstrated the most potent cytotoxic effects on breast cancer MDA-MB-231 cells. Compared with estrogen receptor (ER)-positive or HER2-overexpressing breast cancer cells, DMC demonstrated the most efficient cytotoxic effects on triple-negative breast cancer (TNBC) cells. However, nonmalignant MCF-10A cells were unaffected by DMC treatment. The study showed that DMC activated AMPK in TNBC cells. Once activated, AMPK inhibited eukaryotic initiation factor 4E-binding protein-1 (4E-BP1) signaling and mRNA translation via mammalian target of rapamycin (mTOR) and decreased the activity and/or expression of lipogenic enzymes, such as fatty acid synthase (FASN) and acetyl-CoA carboxylase (ACC). DMC also targeted multiple AMPK downstream pathways. Among these, the dephosphorylation of Akt is noteworthy because it circumvents the feedback activation of Akt that results from mTOR inhibition. Moreover, DMC suppressed LPS-induced IL-6 production, thereby blocking subsequent Stat3 activation. In addition, DMC also sustained epidermal growth factor receptor (EGFR) activation by suppressing the phosphatases, PP2a and SHP-2.
CONCLUSIONS:
These results suggest that DMC is a potent AMPK activator that acts through a broad spectrum of anti-TNBC activities. | | J Agric Food Chem. 2012 Aug 29;60(34):8427-34. | | Demethoxycurcumin modulates prostate cancer cell proliferation via AMPK-induced down-regulation of HSP70 and EGFR.[Pubmed: 22849866] | Curcumin (Cur), Demethoxycurcumin (DMC), and bisDemethoxycurcumin (BDMC) are major forms of curcuminoids found in the rhizomes of turmeric.
METHODS AND RESULTS:
This study examined the effects of three curcuminoid analogues on prostate cancer cells. The results revealed that DMC demonstrated the most efficient cytotoxic effects on prostate cancer PC3 cells. DMC activated AMPK and in turn decreased the activity and/or expression of lipogenic enzymes, such as fatty acid synthase (FASN) and acetyl-CoA carboxylase (ACC). AICAR, an AMPK activator, and DMC down-regulated heat shock protein (HSP) 70 and increased the activity of the pro-apoptotic effector, caspase-3. In addition, DMC sustained epidermal growth factor receptor (EGFR) activation by suppressing the phosphatases PP2a and SHP-2. DMC also increased the interaction between EGFR and Cbl and induced the tyrosine phosphorylation of Cbl.
CONCLUSIONS:
The results suggest that DMC may have antitumor effects on prostate cancer cells via AMPK-induced down-regulation of HSP70 and EGFR. | | Asian Pac J Cancer Prev . 2014;15(4):1807-10. | | Demethoxycurcumin from Curcuma longa rhizome suppresses iNOS induction in an in vitro inflamed human intestinal mucosa model[Pubmed: 24641413] | | It is known that inducible nitric oxide synthase (iNOS)/nitric oxide (NO) plays an integral role during intestinal inflammation, an important factor for colon cancer development. Natural compounds from Curcuma longa L. (Zingiberaceae) have long been a potential source of bioactive materials with various beneficial biological functions. Among them, a major active curcuminoid, Demethoxycurcumin (DMC) has been shown to possess anti-inflammatory properties in lipopolysaccharide (LPS)-activated macrophages or microglia cells. However, the role of DMC on iNOS expression and NO production in an in vitro inflamed human intestinal mucosa model has not yet been elucidated. This study concerned inhibitory effects on iNOS expression and NO production of DMC in inflamed human intestinal Caco-2 cells. An in vitro model was generated and inhibitory effects on NO production of DMC at 65 μM for 24-96 h were assessed by monitoring nitrite levels. Expression of iNOS mRNA and protein was also investigated. DMC significantly decreased NO secretion by 35-41% in our inflamed cell model. Decrease in NO production by DMC was concomitant with down-regulation of iNOS at mRNA and protein levels compared to proinflammatory cytokine cocktail and LPS-treated controls. Mechanism of action of DMC may be partly due to its potent inhibition of the iNOS pathway. Our findings suggest that DMC may have potential as a therapeutic agent against inflammation-related diseases, especially in the gut. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)