| Structure Identification: |
| Bioorg Med Chem Lett. 2009 Jun 1;19(11):3036-40. | | Synthesis of aristolactam analogues and evaluation of their antitumor activity.[Pubmed: 19394218] | A series of natural aristolactams and their analogues have been prepared and evaluated for antitumor activity against human cancer cells, including multi-drug resistant cell lines.
METHODS AND RESULTS:
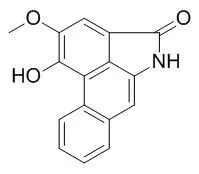
Naturally occurring aristolactams, such as aristolactam BII (cepharanone B), aristolactam BIII, Aristolactam FI (piperolactam A), N-methyl piperolactam A, and sauristolactam showed moderate antitumor activities in selected cell lines.
However, several synthetic aristolactam derivatives exhibited potent antitumor activities against a broad array of cancer cell lines with GI(50) values in the submicromolar range. | | Org Lett. 2008 Aug 21;10(16):3543-6. | | Total synthesis of aristolactams via a one-pot suzuki-miyaura coupling/aldol condensation cascade reaction.[Pubmed: 18642834] |
METHODS AND RESULTS:
A direct one-pot synthesis of phenanthrene lactams, which employs a Suzuki-Miyaura coupling/aldol condensation cascade reaction of isoindolin-1-one with 2-formylphenylboronic acid, has been developed. The approach is used to efficiently produce a number of natural aristolactams, such as aristolactam BII (cepharanone B), aristolactam BIII, Aristolactam FI (piperolactam A), N-methyl piperolactam A, and sauristolactam. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)