| Kinase Assay: |
| Helv. Chim. Acta, 2002, 85(2):678-88. | | Pregnane-Type Steroidal Alkaloids of Sarcococca saligna: A New Class of Cholinesterase Inhibitors[Reference: WebLink] |
METHODS AND RESULTS:
The structures of the new alkaloids salignenamide C (1), salignenamide D (2), 2b-hydroxyepipachysamine D (3), salignenamide E (4), and salignenamide F (5) were elucidated with the help of modern spectroscopic techniques, while the known alkaloids axillarine C (6), axillarine F (7), sarcorine (8), N 3 -demethylsaracodine (9), saligcinnamide (10), salignenamide A (11), vaganine A (12), Axillaridine A (13), sarsalignone (14), and sarsalignenone (15) were identified by comparing their spectral data with those reported earlier. Inhibition of electric-eel acetylcholinesterase (EC 3.1.1.7) and horse-serum butyrylcholinesterase (EC 3.1.1.8) by alkaloids 1 ± 15 were investigated.
CONCLUSIONS:
These new cholinesterase inhibitors may act as potential leads in the discovery of clinically useful inhibitors for nervous-system disorders, particularly by reducing memory deficiency in Alzheimer×s disease patients by potentiating and effecting the cholinergic transmission process. |
|
| Structure Identification: |
| J Enzyme Inhib Med Chem. 2009 Oct;24(5):1101-5. | | Molecular dynamics simulation of Axillaridine-A: a potent natural cholinesterase inhibitor.[Pubmed: 19555175] |
Molecular Dynamics (MD) simulations were carried out for human acetylcholinesterase (hAChE) and its complex with Axillaridine A, in order to dynamically explore the active site of the protein and the behaviour of the ligand at the peripheral binding site.
METHODS AND RESULTS:
Simulation of the enzyme alone showed that the active site of AChE is located at the bottom of a deep and narrow cavity whose surface is lined with rings of aromatic residues while Tyr72 is almost perpendicular to the Trp286, which is responsible for stable pi -pi interactions. The complexation of AChE with Axillaridine A, results in the reduction of gorge size due to interaction between the ligand and the active site residues. The gorge size was determined by the distance between the center of mass of Glu81 and Trp286. As far as the geometry of the active site is concerned, the presence of ligand in the active site alters its specific conformation, as revealed by stable hydrogen bondings established between amino acids. With the increasing interaction between ligand and the active amino acids, size of the active site of the complex decreases with respect to time.
CONCLUSIONS:
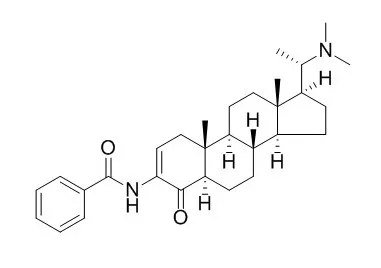
Axillaridine A, forms stable pi -pi interactions with the aromatic ring of Tyr124 that results in inhibition of catalytic activity of the enzyme.
This pi -pi interaction keeps the substrate stable at the edge of the catalytic gorge by inhibiting its catalytic activity. The MD results clearly provide an explanation for the binding pattern of bulky steroidal alkaloids at the active site of AChE. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)