| In vitro: |
| Journal of Shenyang Pharmaceutical University, 2007,24(8) :474-8. | | Cyclic dipeptide constituents from the mangrove fungus Penicillium oxalicum(No.092007).[Reference: WebLink] | To study the metabolites of mangrove fungus Penicillium oxalicum from the south China sea and search for new anti-tumor compounds.
METHODS AND RESULTS:
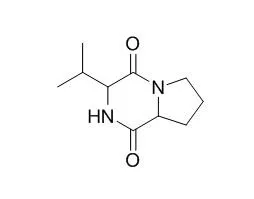
Compounds were isolated by silica gel,ODS column chromatography,Sephadex LH-20 column chromatography and reversed-phase HPLC purification.Structural elucidation was achieved by physico-chemical constants and spectroscopic analysis.MTT assay was used to evaluate the bioactivities in vitro. Six cyclic dipeptides were isolated from the acetone extracts of mycelium of fungus and elucidated as cyclo-(Phe-Ile)(1),cyclo-(Phe-Val)(3),cyclo-(Ile-Leu)(3),cyclo-(Val-Val)(4),Cyclo(Pro-Val)(5),cyclo-(Pro-Gly)(6).Coumpouds 2,3 and 5 could evidently inhibit the growth of cancer cell lines HepG Ⅱ and LNCaP at the concentration of 50 μg·mL-1.
CONCLUSIONS:
All of Compounds are obtained from the marine fungus for the fist time.Compounds 2,3 and 5 show cytotoxic activity in vitro. | | Tetrahedron Lett.,2002, 33(51):6545-8. | | Antifungal cyclopeptides from Halobacillus litoralis YS3106 of marine origin.[Reference: WebLink] |
METHODS AND RESULTS:
Three new cyclopeptides, including halolitoralin A (a cyclic hexapeptide), halolitoralin B and C (two cyclic tetrapeptides), together with three known cyclic dipeptides, Cyclo(Pro-Val), cyclo(Pro-Leu) and cyclo(Ile-Val) were isolated from the ferment broth of a marine sediment-derived Halobacillus litoralis YS3106.
CONCLUSIONS:
The cyclopeptides show surprisingly simple architectures with highly repeated residue units, which showed moderate antifungal and weak antitumor activities in vitro. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)