| In vitro: |
| Arterioscler Thromb Vasc Biol. 2005 Jan;25(1):155-60. | | Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction.[Pubmed: 15499042] | Heme oxygenase-1 (HO-1), the rate-limiting enzyme of heme degradation, has recently been considered to have protective roles against various pathophysiological conditions. Since we demonstrated that HO-1 overexpression inhibits atherosclerotic formation in animal models, we examined the effect of HO modulation on proinflammatory cytokine production, endothelial NO synthase (eNOS) expression, and endothelium-dependent vascular relaxation responses.
METHODS AND RESULTS:
After HO-1 induction by heme arginate (HA), vascular endothelial cell cultures were exposed to oxidized low-density lipoprotein (oxLDL) or tumor necrosis factor-alpha (TNF-alpha). HA pretreatment significantly attenuated the production of vascular cell adhesion molecule-1, monocyte chemotactic protein-1, and macrophage colony-stimulating factor, suggesting that HO-1 induction attenuates proinflammatory responses. In addition, HO-1 overexpression also alleviated endothelial dysfunction as judged by restoration of attenuated eNOS expression after exposure to oxLDL and TNF-alpha. Importantly, impaired endothelium-dependent vascular relaxation responses in thoracic aortic rings from high-fat-fed LDL receptor knockout mice were also improved. These effects were observed by treatment with Bilirubin not by carbon monoxide.
CONCLUSIONS:
These results suggest that the antiatherogenic properties of HO-1 may be mediated predominantly through the action of Bilirubin by inhibition of vascular endothelial activation and dysfunction in response to proinflammatory stresses. | | Science. 1987 Feb 27;235(4792):1043-6. | | Bilirubin is an antioxidant of possible physiological importance.[Pubmed: 3029864] |
METHODS AND RESULTS:
Bilirubin, the end product of heme catabolism in mammals, is generally regarded as a potentially cytotoxic, lipid-soluble waste product that needs to be excreted. However, it is here that Bilirubin, at micromolar concentrations in vitro, efficiently scavenges peroxyl radicals generated chemically in either homogeneous solution or multilamellar liposomes. The antioxidant activity of Bilirubin increases as the experimental concentration of oxygen is decreased from 20% (that of normal air) to 2% (physiologically relevant concentration). Furthermore, under 2% oxygen, in liposomes, Bilirubin suppresses the oxidation more than alpha-tocopherol, which is regarded as the best antioxidant of lipid peroxidation.
CONCLUSIONS:
The data support the idea of a "beneficial" role for Bilirubin as a physiological, chain-breaking antioxidant. | | J Thromb Haemost . 2015 Jun;13(6):1064-72. | | Unconjugated bilirubin inhibits proteolytic cleavage of von Willebrand factor by ADAMTS13 protease[Pubmed: 25782102] | | Abstract
Background: Bilirubin is a yellow breakdown product of heme catabolism. Increased serum levels of unconjugated Bilirubin are conditions commonly seen in premature neonates and adults with acute hemolysis including thrombotic microangiopathy. Previous studies have shown that unconjugated Bilirubin lowers plasma ADAMTS13 activity, but the mechanism is not fully understood.
Objectives: The study is to determine whether unconjugated Bilirubin directly inhibits the cleavage of von Willebrand factor (VWF) and its analogs by ADAMTS13.
Methods: Fluorogenic, surface-enhanced laser desorption/ionization time-of-flight mass spectrometric assay, and Western blotting analyses were used to address this question.
Results: Unconjugated Bilirubin inhibits the cleavage of F485-rVWF73-H, D633-rVWF73-H, and GST-rVWF71-11K by ADAMTS13 in a concentration-dependent manner with a half-maximal inhibitory concentration of ~13, ~70, and ~17 μmol L(-1) , respectively. Unconjugated Bilirubin also dose-dependently inhibits the cleavage of multimeric VWF by ADAMTS13 under denaturing conditions. The inhibitory activity of Bilirubin on the cleavage of D633-rVWF73-H and multimeric VWF, but not F485-rVWF73-H, was eliminated after incubation with Bilirubin oxidase that converts Bilirubin to biliverdin. Furthermore, plasma ADAMTS13 activity in patients with hyperBilirubinemia increased after treatment with Bilirubin oxidase.
Conclusions: Unconjugated Bilirubin directly inhibits ADAMTS13's ability to cleave both peptidyl and native VWF substrates in addition to its interference with certain fluorogenic assays. Our findings may help proper interpretation of ADAMTS13 results under pathological conditions. Whether elevated serum unconjugated Bilirubin has prothrombotic effect in vivo remains to be determined in our future study.
Keywords: ADAMTS13 protein, human; hyperBilirubinemia; thrombosis; thrombotic microangiopathies; von Willebrand factor. | | J Nat Prod . 2013 Oct 25;76(10):1958-65. | | Bilirubin and related tetrapyrroles inhibit food-borne mutagenesis: a mechanism for antigenotoxic action against a model epoxide[Pubmed: 24156291] | | Abstract
Bilirubin exhibits antioxidant and antimutagenic effects in vitro. Additional tetrapyrroles that are naturally abundant were tested for antigenotoxicity in Salmonella. Un-/conjugated Bilirubin (1 and 2), biliverdin (4), Bilirubin and biliverdin dimethyl esters (3 and 5), stercobilin (6), urobilin (7), and protoporphyrin (8) were evaluated at physiological concentrations (0.01-2 μmol/plate; 3.5-714 μM) against the metabolically activated food-borne mutagens aflatoxin B1 (9) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (10). Compound 8 most effectively inhibited the mutagenic effects of 9 in strain TA102 and 10 in TA98. Compound 7 inhibited 9-induced mutagenesis in strain TA98 most effectively, while 1 and 4 were promutagenic in this strain. This is likely due to their competition with mutagens for phase-II detoxification. Mechanistic investigations into antimutagenesis demonstrate that tetrapyrroles react efficiently with a model epoxide of 9, styrene epoxide (11), to form covalent adducts. This reaction is significantly faster than that of 11 with guanine. Hence, the evaluated tetrapyrroles inhibited genotoxicity induced by poly-/heterocyclic amines found in foods, and novel evidence obtained in the present investigation suggests this may occur via chemical scavenging of genotoxic metabolites of the mutagens investigated. This may have important ramifications for maintaining health, especially with regard to cancer prevention. |
|
| In vivo: |
| Proc Natl Acad Sci U S A. 2009 Mar 31;106(13):5171-6. | | Bilirubin and glutathione have complementary antioxidant and cytoprotective roles.[Pubmed: 19286972] |
METHODS AND RESULTS:
Glutathione (GSH) and Bilirubin are prominent endogenous antioxidant cytoprotectants. Despite tissue levels that are thousands of times lower than GSH, Bilirubin is effective because of the biosynthetic cycle wherein it is generated from biliverdin by biliverdin reductase (BVR). When Bilirubin acts as an antioxidant, it is oxidized to biliverdin, which is immediately reduced by BVR to Bilirubin. Why does the body employ both of these 2 distinct antioxidant systems?
CONCLUSIONS:
We show that the water-soluble GSH primarily protects water soluble proteins, whereas the lipophilic Bilirubin protects lipids from oxidation. Mice with deletion of heme oxygenase-2, which generates biliverdin, display greater lipid than protein oxidation, while the reverse holds for GSH depletion. RNA interference depletion of BVR increases oxidation of lipids more than protein. Depletion of BVR or GSH augments cell death in an oxidant-specific fashion. | | FASEB J. 2005 Nov;19(13):1890-2 | | Bilirubin decreases nos2 expression via inhibition of NAD(P)H oxidase: implications for protection against endotoxic shock in rats.[Pubmed: 16129699 ] | We investigated a possible beneficial role for Bilirubin, one of the products of heme degradation by the cytoprotective enzyme heme oxygenase-1 in counteracting Escherichia coli endotoxin-mediated toxicity.
METHODS AND RESULTS:
Homozygous jaundice Gunn rats, which display high plasma Bilirubin levels due to deficiency of glucuronyl transferase activity, and Sprague-Dawley rats subjected to sustained exogenous Bilirubin administration were more resistant to endotoxin (LPS)-induced hypotension and death compared with nonhyperBilirubinemic rats. LPS-stimulated production of nitric oxide (NO) was significantly decreased in hyperBilirubinemic rats compared with normal animals; this effect was associated with reduction of inducible NO synthase (NOS2) expression in renal, myocardial, and aortic tissues. Furthermore, NOS2 protein expression and activity were reduced in murine macrophages stimulated with LPS and preincubated with Bilirubin at concentrations similar to that found in the serum of hyperBilirubinemic animals. This effect was secondary to inhibition of NAD(P)H oxidase since 1) inhibition of NAD(P)H oxidase attenuated NOS2 induction by LPS, 2) Bilirubin decreased NAD(P)H oxidase activity in vivo and in vitro, and 3) down-regulation of NOS2 by Bilirubin was reversed by addition of NAD(P)H.
CONCLUSIONS:
These findings indicate that Bilirubin can act as an effective agent to reduce mortality and counteract hypotension elicited by endotoxin through mechanisms involving a decreased NOS2 induction secondary to inhibition of NAD(P)H oxidase. |
|


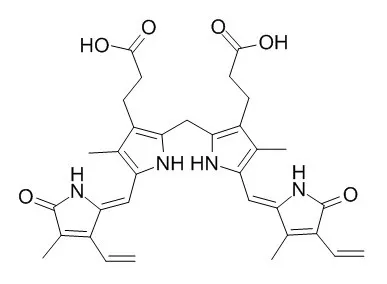



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)