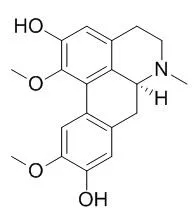
| Description: |
Boldine displays anti-cancer, cytoprotective , anti-oxidant and anti-inflammatory activities. Boldine reduces oxidative stress and improves endothelium-dependent relaxation in aortas of diabetic mice largely through inhibiting ROS overproduction associated with Ang II-mediated BMP4-dependent mechanisms. Boldine may attenuate the catecholamine oxidation-induced brain mitochondrial dysfunction and decrease the dopamine-induced death of PC12 cells through a scavenging action on reactive oxygen species and inhibition of melanin formation and thiol oxidation.
|
| Targets: |
FXR | ROS | GSK-3 | Akt | ERK | NOS | Calcium Channel |
| In vitro: |
| Urol Oncol. 2014 Jan;32(1):36.e1-9. | | Boldine induces cell cycle arrest and apoptosis in T24 human bladder cancer cell line via regulation of ERK, AKT, and GSK-3β.[Pubmed: 24239461 ] | | The objective of the present study was to evaluate the effect and underlying mechanisms of Boldine, an aporphine alkaloid of Peumus boldus, on bladder cancer proliferation and cell death.
METHODS:
Sulforhodamine B assay, Tetrazolium reduction assay, Flow Cytometry Analysis, Ecto-5'-nucleotidase activity and Western blot assay were performed.
RESULTS:
The results showed that Boldine was able to reduce cell viability and cell proliferation in T24 cells. In addition, Boldine arrests the cell cycle at G2/M-phase and cause cell death by apoptosis. Boldine-induced inhibition of cell growth and cell cycle arrest appears to be linked to inactivation of extracellular signal-regulated kinase protein (ERK). Additionally, the efficacy of Boldine in apoptosis-induced in T24 cells is correlated with modulation of AKT (inactivation) and glycogen synthase kinase-3β (GSK-3β) (activation) proteins.
CONCLUSIONS:
The present findings may, in part, explain the therapeutic effects of Boldine for treatment of urinary bladder cancer. | | Neurotoxicology. 2008 Nov;29(6):1136-40. | | Antioxidant and pro-oxidant properties of boldine on hippocampal slices exposed to oxygen-glucose deprivation in vitro.[Pubmed: 18590764 ] | Boldine is one of the most potent natural antioxidants and displays some important pharmacological activities, such as cytoprotective and anti-inflammatory activities, which may arise from its free radical scavenging properties.
METHODS AND RESULTS:
Given that the pathogenesis of brain ischemia/reperfusion has been associated with an excessive generation of oxygen free radicals, the aim of this study was to evaluate the neuroproperties of Boldine using hippocampal slices from Wistar rats exposed to oxygen and glucose deprivation (OGD), followed by reoxygenation, to mimic an ischemic condition. The OGD ischemic condition significantly impaired cellular viability, increased lactate dehydrogenase (LDH) leakage and increased free radical generation. In non-OGD slices, incubation with 100microM Boldine significantly increased LDH released into incubation media and decreased mitochondrial activity, suggesting an increase of tissue damage caused by Boldine. However, slices incubated with 10microM Boldine during and after OGD exposure had significantly increased cellular viability with no effect on cell damage. Total reactive antioxidant potential (TRAP) levels measured for this alkaloid showed an antioxidant potential three times higher than Trolox, which acts as a peroxyl radical scavenger. Moreover, Boldine prevented the increase in lipoperoxidation levels induced by ischemia, but higher concentrations potentiated this parameter.
CONCLUSIONS:
These results confirm the potent antioxidant properties of this alkaloid, and add evidence to support the need for further investigations in order to confirm the potential pro-oxidant effects of Boldine at higher doses. |
|
| In vivo: |
| Toxicol Appl Pharmacol. 2015 May 15;285(1):12-22. | | Boldine enhances bile production in rats via osmotic and farnesoid X receptor dependent mechanisms.[Pubmed: 25771127] | Boldine, the major alkaloid from the Chilean Boldo tree, is used in traditional medicine to support bile production, but evidence to support this function is controversial. We analyzed the choleretic potential of Boldine, including its molecular background.
METHODS AND RESULTS:
The acute- and long-term effects of Boldine were evaluated in rats either during intravenous infusion or after 28-day oral treatment. Infusion of Boldine instantly increased the bile flow 1.4-fold in healthy rats as well as in animals with Mrp2 deficiency or ethinylestradiol induced cholestasis. This effect was not associated with a corresponding increase in bile acid or glutathione biliary excretion, indicating that the effect is not related to stimulation of either bile acid dependent or independent mechanisms of bile formation and points to the osmotic activity of Boldine itself. We subsequently analyzed bile production under conditions of changing biliary excretion of Boldine after bolus intravenous administration and found strong correlations between both parameters. HPLC analysis showed that bile concentrations of Boldine above 10 μM were required for induction of choleresis. Importantly, long-term pretreatment, when the bile collection study was performed 24-h after the last administration of Boldine, also accelerated bile formation despite undetectable levels of the compound in bile. The effect paralleled upregulation of the Bsep transporter and increased biliary clearance of its substrates, bile acids. We consequently confirmed the ability of Boldine to stimulate the Bsep transcriptional regulator, FXR receptor.
CONCLUSIONS:
In conclusion, our study clarified the mechanisms and circumstances surrounding the choleretic activity of Boldine. | | Br J Pharmacol. 2013 Nov;170(6):1190-8. | | Boldine improves endothelial function in diabetic db/db mice through inhibition of angiotensin II-mediated BMP4-oxidative stress cascade.[Pubmed: 23992296] | Boldine is a potent natural antioxidant present in the leaves and bark of the Chilean boldo tree. Here we assessed the protective effects of Boldine on endothelium in a range of models of diabetes, ex vivo and in vitro.
METHODS AND RESULTS:
Vascular reactivity was studied in mouse aortas from db/db diabetic and normal mice. Reactive oxygen species (ROS) production, angiotensin AT1 receptor localization and protein expression of oxidative stress markers in the vascular wall were evaluated by dihydroethidium fluorescence, lucigenin enhanced-chemiluminescence, immunohistochemistry and Western blot respectively. Primary cultures of mouse aortic endothelial cells, exposed to high concentrations of glucose (30 mmol L(-1) ) were also used.
Oral treatment (20 mg kg(-1) day(-1) , 7 days) or incubation in vitro with Boldine (1 μmol L(-1) , 12 h) enhanced endothelium-dependent aortic relaxations of db/db mice. Boldine reversed impaired relaxations induced by high glucose or angiotensin II (Ang II) in non-diabetic mouse aortas while it reduced the overproduction of ROS and increased phosphorylation of eNOS in db/db mouse aortas. Elevated expression of oxidative stress markers (bone morphogenic protein 4 (BMP4), nitrotyrosine and AT1 receptors) were reduced in Boldine-treated db/db mouse aortas. Ang II-stimulated BMP4 expression was inhibited by Boldine, tempol, noggin or losartan. Boldine inhibited high glucose-stimulated ROS production and restored the decreased phosphorylation of eNOS in mouse aortic endothelial cells in culture.
CONCLUSIONS:
Boldine reduced oxidative stress and improved endothelium-dependent relaxation in aortas of diabetic mice largely through inhibiting ROS overproduction associated with Ang II-mediated BMP4-dependent mechanisms. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)