| Cell Research: |
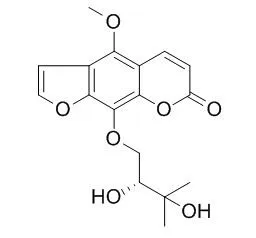
| Br J Pharmacol. 2011 Jan;162(2):441-51. | | Byakangelicin induces cytochrome P450 3A4 expression via transactivation of pregnane X receptors in human hepatocytes.[Pubmed: 20942813] | Byakangelicin is found in extracts of the root of Angelica dahurica, used in Korea and China as a traditional medicine to treat colds, headache and toothache. As Byakangelicin can inhibit the effects of sex hormones, it may increase the catabolism of endogenous hormones. Therefore, this study investigated the effects of Byakangelicin on the cytochrome P450 isoform cytochrome (CY) P3A4 in human hepatocytes.
METHODS AND RESULTS:
Cultures of human hepatocytes and a hepatoma cell line (Huh7 cells) were used. mRNA and protein levels were measured by quantitative reverse transcription-polymerase chain reaction and Western blot. Plasmid constructs and mutants were prepared by cloning and site-directed mutagenesis. Reporter (luciferase) activity was determined by transient co-transfection experiments.
In human primary hepatocytes, Byakangelicin markedly induced the expression of CYP3A4 both at the mRNA level (approximately fivefold) and the protein level (approximately threefold) but did not affect expression of human pregnane X receptor (hPXR). In reporter assays, Byakangelicin activated CYP3A4 promoter in a concentration-dependent manner (EC₅₀ = 5 μM), and this activation was enhanced by co-transfection with hPXR. Further reporter assays demonstrated that the eNR4 binding element in the CYP3A4 promoter was required for the transcriptional activation of CYP3A4 by Byakangelicin.
CONCLUSIONS:
Byakangelicin induced expression and activity of CYP3A4 in human hepatocytes. This induction was achieved by the transactivation of PXR and not by increased expression of PXR. Therefore, Byakangelicin is likely to increase the expression of all PXR target genes (such as MDR1) and induce a wide range of drug-drug interactions. |
|
| Structure Identification: |
| Arch Pharm Res. 2003 Aug;26(8):606-11. | | Identification of new urinary metabolites of byakangelicin, a component of Angelicae dahuricae Radix, in rats.[Pubmed: 12967195] | Byakangelicin, 9-(2,3-dihydroxy-2-methylbutoxy)-4-methoxy-7H-furo[3,2-g][l]benzopyran-7-one (BKG), a component of Angelicae dahuricae Radix, is considered to be an inhibitor of aldose reductase for the treatment of diabetic cataract.
METHODS AND RESULTS:
An analytical method for the isolation of BKG developed by high-performance liquid chromatography has been reported. No literature on the metabolism of BKG, however, has been found. With the purpose of identifying new metabolites of BKG, BKG (100 mg/kg) was orally administered to Sprague-Dawley rats via a gavage. Using a metabolic cage, urine was collected for 24 h, and the urine samples were extracted by liquid-liquid extraction. For structural identification of new urinary metabolites of BKG, various instrumental analyses were conducted by gas-chromatography/mass spectrometry, high-performance liquid chromatography/diode array detector, liquid chromatography/mass spectroscopy with thermospray interface and 1H nuclear magnetic resonance spectroscopy. Two metabolites produced from the O-demethylation or O-dealkylation of BKG were newly identified, and another new but unknown metabolite was assumed to be the hydroxylated form of BKG.
CONCLUSIONS:
These results indicate that the major metabolic products of BKG are formed by O-demethylation or O-dealkylation of BKG side chains. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)