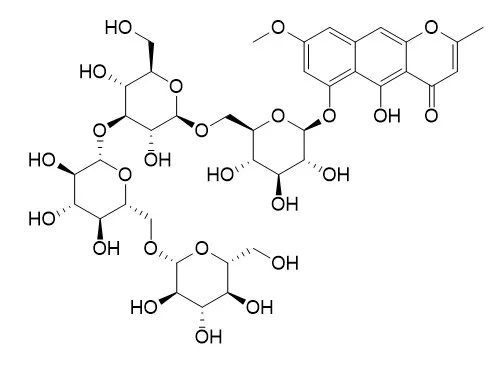
A number of nature-derived biologically active compounds comprise glycosides. In some cases, the glycosidic residue is needed for bioactivity; however, in other cases, glycosylation just improves some pharmacokinetic/dynamic parameters. The patterns of protein tyrosine phosphatase 1B (PTP1B) and human monoamine oxidase A (hMAO-A) inhibition by rubrofusarin 6-O-β-d-glucopyranoside (1), rubrofusarin 6-O-β-d-gentiobioside (2), rubrofusarin triglucoside (3), and Cassiaside B2 (4) were compared with the aglycone, rubrofusarin, isolated from Cassia obtusifolia seeds.
METHODS AND RESULTS:
Rubrofusarin showed potent inhibition against the PTP1B enzyme (IC50; 16.95 ± 0.49 μM), and its glycosides reduced activity (IC50; 87.36 ± 1.08 μM for 1 and >100 μM for 2-4) than did the reference drug, ursolic acid (IC50; 2.29 ± 0.04 μM). Similarly, in hMAO-A inhibition, rubrofusarin displayed the most potent activity with an IC50 value of 5.90 ± 0.99 μM, which was twice better than the reference drug, deprenyl HCl (IC50; 10.23 ± 0.82 μM). An enzyme kinetic and molecular docking study revealed rubrofusarin to be a mixed-competitive inhibitor of both these enzymes. In a western blot analysis, rubrofusarin increased glucose uptake significantly and decreased the PTP1B expression in a dose-dependent manner in insulin-resistant HepG2 cells, increased the expression of phosphorylated protein kinase B (p-Akt) and phosphorylated insulin receptor substrate-1 (p-IRS1) (Tyr 895), and decreased the expression of glucose-6-phosphatase (G6Pase) and phosphoenol pyruvate carboxykinase (PEPCK), key enzymes of gluconeogenesis.
CONCLUSIONS:
Our overall results show that glycosylation retards activity; however, it reduces toxicity. Thus, Cassia seed as functional food and rubrofusarin as a base can be used for the development of therapeutic agents against comorbid diabetes and depression. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)