| Structure Identification: |
| The Alkaloids: Chemistry and Pharmacology, 1985,26: 53–87. | | Chapter 2 Sulfur-Containing Alkaloids[Reference: WebLink] |
METHODS AND RESULTS:
A great variety of structures with different chromophores were recognized together with different numbers of sulfur atoms (one to four), which could be placed either in the side chain or in the ring. The increasing interest in this group of alkaloids is connected with new structures and chemical properties as well as with pharmacological activity. The sulfur-containing alkaloids may be classified in many ways. This chapter provides a guide for the classification, which denotes the number of sulfur atoms and their presence in the side chain or in the ring.
CONCLUSIONS:
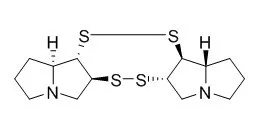
Alkaloids with one sulfur atom in the side chain are described, which include erysothiovine and erysothiopine, 4-methylthiocanthin-6-one, zapotidine, planchonellin, japindine, and resedinine and related alkaloids. Alkaloids with one sulfur atom in the ring include chuangxinmycin, alkaloid TM-64, ferrithiocin, myxothiazol, and xylostosidine and its S-oxides. The chapter also describes alkaloids with more than one sulfur atom, such as brugine, gliotoxin, gliovirine, sporidesmins, sirodesmins, hyalodendrin, and Cassipourine and related alkaloids. sulfur-containing alkaloids of peptide structure are also discussed. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)