| Kinase Assay: |
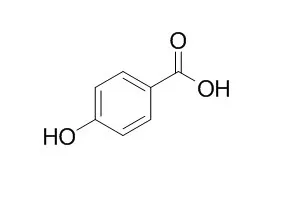
| Cancer Lett. 2014 Feb 1;343(1):134-46. | | Antiproliferative and proapoptotic activities of 4-hydroxybenzoic acid-based inhibitors of histone deacetylases.[Pubmed: 24080339] | Histone acetyltransferases (HATs) and histone deacetylases (HDACs) regulate cellular processes by modifying the acetylation status of many proteins. Pathologically altered HDAC activity contributes to cancer development and thus characterization of novel acetylation modulators is important for future anti-cancer therapies.
METHODS AND RESULTS:
In this study, we identified three novel 4-Hydroxybenzoic acid derivatives as pan-HDAC inhibitors that increased protein acetylation levels, arrested cell cycle progression and triggered apoptotic cell death, without affecting viability of normal cells.
CONCLUSIONS:
Our data support the potential of 4-Hydroxybenzoic acid derivatives as pan-HDAC inhibitors with anticancer properties. | | Mol Plant Microbe Interact. 2013 Oct;26(10):1239-48. | | The rice bacterial pathogen Xanthomonas oryzae pv. oryzae produces 3-hydroxybenzoic acid and 4-hydroxybenzoic acid via XanB2 for use in xanthomonadin, ubiquinone, and exopolysaccharide biosynthesis.[Pubmed: 23718125] | Xanthomonas oryzae pv. oryzae, the causal agent of rice bacterial blight, produces membrane-bound yellow pigments, referred to as xanthomonadins. Xanthomonadins protect the pathogen from photodamage and host-induced perioxidation damage. They are also required for epiphytic survival and successful host plant infection.
METHODS AND RESULTS:
Here, we show that XanB2 encoded by PXO_3739 plays a key role in xanthomonadin and coenzyme Q8 biosynthesis in X. oryzae pv. oryzae PXO99A. A xanB2 deletion mutant exhibits a pleiotropic phenotype, including xanthomonadin deficiency, producing less exopolysaccharide (EPS), lower viability and H2O2 resistance, and lower virulence. We further demonstrate that X. oryzae pv. oryzae produces 3-hydroxybenzoic acid (3-HBA) and 4-Hydroxybenzoic acid (4-HBA) via XanB2. 3-HBA is associated with xanthomonadin biosynthesis while 4-HBA is mainly used as a precursor for coenzyme Q (CoQ)8 biosynthesis. XanB2 is the alternative source of 4-HBA for CoQ8 biosynthesis in PXO99A. These findings suggest that the roles of XanB2 in PXO99A are generally consistent with those in X. campestris pv. campestris. The present study also demonstrated that X. oryzae pv. oryzae PXO99A has evolved several specific features in 3-HBA and 4-HBA signaling. First, our results showed that PXO99A produces less 3-HBA and 4-HBA than X. campestris pv. campestris and this is partially due to a degenerated 4-HBA efflux pump. Second, PXO99A has evolved unique xanthomonadin induction patterns via 3-HBA and 4-HBA. Third, our results showed that 3-HBA or 4-HBA positively regulates the expression of gum cluster to promote EPS production in PXO99A.
CONCLUSIONS:
Taken together, the results of this study indicate that XanB2 is a key metabolic enzyme linking xanthomonadin, CoQ, and EPS biosynthesis, which are collectively essential for X. oryzae pv. oryzae pathogenesis. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)