| Structure Identification: |
| J Nat Prod. 1998 May;61(5):629-32. | | Antitumor-promoting naphthoquinones from Catalpa ovata.[Pubmed: 9599262] |
METHODS AND RESULTS:
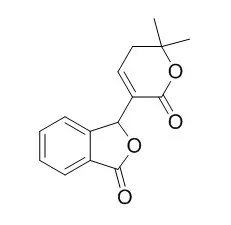
Bioassay-directed fractionation of an extract of the stem-bark of Catalpa ovata led to the isolation of three new naphthoquinones: 8-methoxydehydroiso-alpha-lapachone (1), 9-methoxy-4-oxo-alpha-lapachone (2), and (4S,4aR,10R,10aR)-4, 10-dihydroxy-2,2-dimethyl-2,3,4,4alpha,10, 10alpha-hexahydrobenzo[g]chromen-5-one (3), which is a 1,4-reductive form of 6. The known compounds 3-hydroxydehydroiso-alpha-lapachone (4), 4,9-dihydroxy-alpha-lapachone (5), 4-hydroxy-alpha-lapachone (6), and 9-methoxy-alpha-lapachone (7), and Catalpalactone (8) were also isolated. Their structures were elucidated by spectral methods.
CONCLUSIONS:
These compounds all exhibited significant inhibitory activity against 12-O-tetradecanoylphorbol 13-acetate (TPA)-induced Epstein-Barr virus early antigen (EBV-EA) activation in Raji cells. | | Periodical of Ocean University of China, 2012, 42: 127-45. | | An Improved Synthesis and Cytotoxicity Activities of Catalpalactone[Reference: WebLink] |
METHODS AND RESULTS:
An improved synthesis of Catalpalactone was carried out with a total yield of 18% by using 2-formylbenzoic acid as the starting material followed by a four-steps procedure including Wittig reaction,lactonization,retro-Michael addition and a one-pot manipulation to introduce the double bond.The structures of all of the intermediates as well as the stereo-configurations of double bond in compounds 11,12a and 12b were determined by 1H NMR,13C NMR,MS,1H-1H COSY and NOE techniques.This improved synthetic method provides several advantages such as inexpensive and easily available reagents,mild reaction conditions,simple operations,and high yields.
CONCLUSIONS:
Through being evaluated in vitro against a panel of two human tumor cell lines(MCF-7,BxPC3) which were determined by the SRB assays, Catalpalactone displayed good activities against MCF-7 and BxPC3. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)