| Description: |
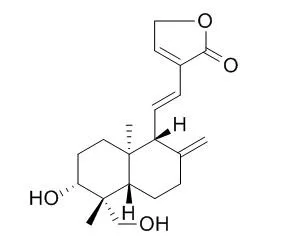
14-Deoxy-11,12-didehydroandrographolide has hypotensive, anti-inflammatory, anti-asthma, and anti-cancer actions, it causes negative chronotropic action and antagonised isoproterenol-induced positive chronotropic actions in a non-competitive and dose-dependent manner. 14-Deoxy-11,12-didehydroandrographolide can effectively ameliorate astrocytic pro-inflammatory reactions and prevent PC12 cell death with different efficacies, it may be candidates for treatment of spinal-cord injury and neurodegeneration. |
| In vitro: |
| J Nat Med. 2014 Apr;68(2):387-94. | | 14-Deoxy-11,12-didehydroandrographolide inhibits proliferation and induces GSH-dependent cell death of human promonocytic leukemic cells.[Pubmed: 24458985] | 14-Deoxy-11,12-didehydroandrographolide (AND2), an analogue of andrographolide, showed more potent cytotoxicity against human promonocytic leukemia (THP-1) cells than adherent cancer cell lines.
METHODS AND RESULTS:
In this study 14-Deoxy-11,12-didehydroandrographolide was isolated from the plant Andrographis paniculata and it was characterized. The antiproliferative effect of 14-Deoxy-11,12-didehydroandrographolide on both adherent (PC-3 and MDAMB) and non-adherent (THP-1 and Jurkat) cancer cell lines was evaluated by MTT assay. The effect of intracellular reduced glutathione (GSH) on 14-Deoxy-11,12-didehydroandrographolide-induced cytotoxicity was studied by conducting cell viability assays on GSH-pretreated cells. The effect of 14-Deoxy-11,12-didehydroandrographolide on the redox status of THP-1 cells was determined by analyzing the endogenous reduced GSH content. Apoptosis induction was confirmed by DNA laddering assay and Western blot analysis using anti-caspase-3 protein antibody. 14-Deoxy-11,12-didehydroandrographolide showed antiproliferative action on both THP-1 and Jurkat cancer cell lines with low IC50 values. Cytotoxicity of AND2 was reversed by GSH pretreatment. AND2 treatment decreased the GSH content by 19.76 % (p < 0.001) in the THP-1 cancer cell line and reduced the cell clumping between the THP-1 cells. Expression of procaspase-3 varied in THP-1 cells during the time course of 14-Deoxy-11,12-didehydroandrographolide treatment. Procaspase-3 expression reached a maximum in treated cells at 32 h and was markedly reduced at 48 h but no procaspase-3 cleavage was observed. The obtained results suggest that 14-Deoxy-11,12-didehydroandrographolide is more effective against leukemia cells. 14-Deoxy-11,12-didehydroandrographolide induced a redox-mediated cell death in THP-1 cells.
CONCLUSIONS:
As 14-Deoxy-11,12-didehydroandrographolide temporarily increased the procaspase-3 expression during treatment, this study encourages the preclinical testing of 14-Deoxy-11,12-didehydroandrographolide against promonocytic leukemia cells in combination with small molecules that directly activate procaspase-3 to caspase-3. | | Life Sci. 2012 Feb 13;90(7-8):257-66. | | Effects of andrographolide and 14-deoxy-11,12-didehydroandrographolide on cultured primary astrocytes and PC12 cells.[Pubmed: 22154981] | To test the effects of andrographolide (AP1) and 14-Deoxy-11,12-didehydroandrographolide (AP2) on pheochromocytoma cell line 12 (PC12) cells in an astrocyte-rich environment.
METHODS AND RESULTS:
The abilities of AP1 and 14-Deoxy-11,12-didehydroandrographolide to reduce the secretion of pro-inflammatory cytokines Interleukin (IL)-1, IL-6, and Tumor necrosis factor (TNF)-α from stimulated astrocytes were tested. In addition, the abilities of AP1 and 14-Deoxy-11,12-didehydroandrographolide to reduce oxidative stress in astrocytes were tested using an oxidative-sensitive fluorescent dye. The reduction of chondroitin sulfate proteoglycan (CSPG) in stimulated astrocytes was tested using the dot blot method. Reduction of H(2)O(2)-induced death was tested in PC12 cells. Astrocyte-conditioned medium (ACM) and TNF-α-stimulated astrocyte-conditioned medium (SACM) were used to assess the effects of 14-Deoxy-11,12-didehydroandrographolide on PC12 cells treated with H(2)O(2). AP1 and 14-Deoxy-11,12-didehydroandrographolide reduced pro-inflammatory cytokines, reactive oxygen species (ROS), and CSPG in TNF-α stimulated astrocytes. AP1 protected H(2)O(2)-treated PC12 cells cultured in ACM. Co-incubation of PC12 cells in H(2)O(2), and ACM collected from AP1 treated astrocytes did not prevent cell death.
CONCLUSIONS:
AP1 and 14-Deoxy-11,12-didehydroandrographolide effectively ameliorated astrocytic pro-inflammatory reactions and prevented PC12 cell death with different efficacies. These compounds may be candidates for treatment of spinal-cord injury and neurodegeneration. |
|
| In vivo: |
| Pharmacol Res. 1998 Dec;38(6):413-7. | | Cardiovascular activity of 14-deoxy-11,12-didehydroandrographolide in the anaesthetised rat and isolated right atria.[Pubmed: 9990649 ] |
METHODS AND RESULTS:
The cardiovascular activity of 14-Deoxy-11,12-didehydroandrographolide (DDA) from Andrographis paniculata (Burm.f.) Nees (Acanthaceae) was elucidated in anaesthetised Sprague-Dawley (SD) rats and isolated rat right atria. In anaesthetised rats, DDA produced significant falls in mean arterial blood pressure (MAP) and heart rate in a dose-dependent manner with the maximum decrease of 37.6 +/- 2.6% and 18.1 +/- 4.8%, respectively. The ED50 value for MAP was 3.43 mmol kg-1. Pharmacological antagonist studies were done using this dose. The hypotensive action of DDA was not mediated through effects on the alpha-adrenoceptor, muscarinic cholinergic and histaminergic receptors, for it was not affected by phentolamine, atropine as well as pyrilamine and cimetidine. However, it seems to work via adrenoceptors, autonomic ganglia receptor and angiotensin-converting enzyme, since the hypotensive effect of DDA was negated or attenuated in the presence of propranolol, hexamethonium and captopril. In the isolated right atria, DDA caused negative chronotropic action and antagonised isoproterenol-induced positive chronotropic actions in a non-competitive and dose-dependent manner.
CONCLUSIONS:
These results further supported the bradycardia-inducing and beta-adrenoceptor antagonistic properties of DDA in vivo. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)