| Cell Research: |
| Biosci Biotechnol Biochem. 2012;76(9):1616-20. | | Antifibrotic activity of diarylheptanoids from Betula platyphylla toward HSC-T6 cells.[Pubmed: 22972321 ] |
METHODS AND RESULTS:
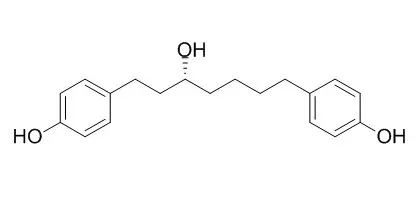
A chemical investigation of the n-butanol fraction of the inner bark of Betula platyphylla led to the isolation of seven diarylhepanoids, (-)-Centrolobol (1), aceroside VII (2), aceroside VIII (3), (3R)-1,7-bis-(4-hydroxyphenyl)-3-heptanol-3-O-[2,6-bis-O-(β-D-apiofuranosyl)-β-D-glucopyranoside (4), 1,7-bis-(4-hydroxyphenyl)-5-hepten-3-one (5), platyphyllone (6) and platyphylloside (7). The antifibrotic effects of these isolates were evaluated with HSC-T6 cells by assessing cell proliferation. Among them, compounds 1, 2, 5 and 6 significantly inhibited the proliferation of HSCs in a dose-dependent manner at concentrations from 10 μM to 100 μM. Compound 5 in particular dramatically decreased the collagen content and increased the Caspase-3/7 activity.
CONCLUSIONS:
Taken together, the antifibrotic activity of B. platyphylla and its constituents might suggest therapeutic potential against liver fibrosis. |
|
| Structure Identification: |
| Records of Natural Products, 2014, 8(1):46-50. | | Cytotoxic Sesquiterpenoids and Diarylheptanoids from the Rhizomes of Curcuma elata Roxb.[Reference: WebLink] |
METHODS AND RESULTS:
The present study was aimed to investigate the chemical constituents of Curcuma elata Roxb. (Zingiberaceae) rhizomes originating in Thailand. Ten sesquiterpenes, germacrone (1), curzerenone (2), isofuranodienone (3), furanodienone (4), curdione (5), neocurdione (6), zederone (7), curcumenone (8), 13-hydroxygermacrone (9) and zedoarondiol (10), and four diarylheptanoids, 3-hydroxy-5-platyphyllone (11), (3S)-1,7-bis(4-hydroxyphenyl)-(6E)-6-hepten-3-ol (12), Centrolobol (13) and (3S)-1-(3,4-dihydroxyphenyl)-7-(4-hydroxyphenyl)-(6E)-6-hepten-3-ol (14) were isolated for the first time from the rhizomes of this plant. The structures of the isolated compounds were identified by comparison of the spectroscopic and physical data with those of the reported values and the stereochemistry at the asymmetric carbon was determined by the modified Mosher's method and, in some cases, was confirmed by comparison of optical rotation data with literature.
CONCLUSIONS:
Compounds 12 and 13 exhibited strong cytotoxic activity against KB cell line, whereas compounds 4, 9 and 12-14 showed strong cytoxicity against NCI-H187 cell line.
|
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)