| In vitro: |
| Molecules. 2014 Dec 15;19(12):21044-65. | | Antiprotozoal activity against Entamoeba histolytica of plants used in northeast Mexican traditional medicine. Bioactive compounds from Lippia graveolens and Ruta chalepensis.[Pubmed: 25517343] | Amoebiasis caused by Entamoeba histolytica is associated with high morbidity and mortality is becoming a major public health problem worldwide, especially in developing countries.
Because of the side-effects and the resistance that pathogenic protozoa build against the standard antiparasitic drugs, e.g., metronidazole, much recent attention has been paid to plants used in traditional medicine around the world in order to find new antiprotozoal agents.
METHODS AND RESULTS:
We collected 32 plants used in Northeast Mexican traditional medicine and the methanolic extracts of these species were screened for antiprotozoal activity against E. histolytica trophozoites using in vitro tests. Only 18 extracts showed a significant inhibiting activity and among them six plant extracts showed more than 80% growth inhibition against E. histolytica at a concentration of 150 μg/mL and the IC50 values of these extracts were determined. Lippia graveolens Kunth and Ruta chalepensis Pers. showed the more significant antiprotozoal activity (91.54% and 90.50% growth inhibition at a concentration of 150 μg/mL with IC50 values of 59.14 and 60.07 μg/mL, respectively).
CONCLUSIONS:
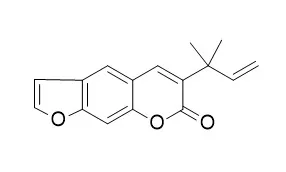
Bioassay-guided fractionation of the methanolic extracts from these two plants afforded carvacrol (1) and Chalepensin (2), respectively, as bioactive compounds with antiprotozoal activity. | | Phytochemistry. 2005 Feb;66(4):487-94. | | Allelochemicals from Stauranthus perforatus, a Rutaceous tree of the Yucatan Peninsula, Mexico.[Pubmed: 15694456 ] | Aqueous leachates and a CHCl3-MeOH (1:1) extract of roots of Stauranthus perforatus showed a significant phytotoxic effect on Amaranthus hypochondriacus and Echinochloa crus-galli.
METHODS AND RESULTS:
Bioassay-directed fractionation of the active organic extract led to the isolation and characterization of ten secondary metabolites, which included two pyranocoumarins [xanthyletin (1) and 3-(1',1'-dimethylallyl)-xanthyletin (2)], four furanocoumarins [Chalepensin (3), ammirin (4), chalepin (5) and 2'-isopropyl-psoralene (6)], two lignans [asarinin (7) and fargesin (8)], one sesquiterpene [4,5-epoxi-beta-caryophyllene (9)], and one alkamide [pellitorine (10)]. From these compounds, 2'-isopropyl-psoralene (6) or anhydromarmesin, is reported for the first time as a natural product, whereas compounds 4-10 are now reported as being present in S. perforatus.
CONCLUSIONS:
Metabolites 1, 3-5 and 10 caused significant inhibition of radicle growth of A. hypochondriacus and E. crus-galli. Furthermore, in a greenhouse experiment the decomposition of the leaves and roots in the soil had a significant inhibitory effect on the growth of weeds. The allelopathic action of the decomposition of roots was evident up to the sixth week of the experiment.
The effect of leaves was comparable to that of DPCA (dimethyl tetrachloroterephthalate), a commercial herbicide. Finally different concentrations of Stauranthus root powder were combined with maize kernels and used to feed corn weevil. The treatments resulted in high mortality of this insect. | | J Agric Food Chem. 1999 May;47(5):2137-40. | | Effect of selected coumarins on spinach chloroplast photosynthesis.[Pubmed: 10552509] |
METHODS AND RESULTS:
Xanthyletin (1), 3-(1',1'-dimethylallyl)xanthyletin (2), and Chalepensin (3), the major coumarins isolated from Stauranthus perforatus, inhibit ATP synthesis from water to methylviologen in spinach thylakoids in a concentration-dependent manner. At low concentration Chalepensin (3) inhibits basal and phosphorylating electron flow from water to K(3)[Fe(CN)(6)] without affecting uncoupled electron flow but accelerating Mg(2 )-ATPase activity.
CONCLUSIONS:
Thus, at low concentration the compound behaves as an energy transfer inhibitor. However, at higher concentrations this coumarin acts as an uncoupler because it enhances basal and phosphorylating electron transfer. On the other hand, coumarins 1 and 2 act as Hill reaction inhibitors, although 2 exhibited also uncoupler properties because it induces stimulation of basal and phosphorylating electron flow from water to ferricyanide. The site of interference of xanthyletin was located at the b(6)f-PC level of the electron transport chain. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)