| Structure Identification: |
| Plant Sci. 2015 Oct;239:106-14. | | Production of dammarane-type sapogenins in rice by expressing the dammarenediol-II synthase gene from Panax ginseng C.A. Mey.[Pubmed: 26398795 ] | Ginsenosides are the main active ingredients in Chinese medicinal ginseng; 2,3-oxidosqualene is a precursor metabolite to ginsenosides that is present in rice. Because rice lacks a key rate-limiting enzyme (Dammarenediol II synthase, DS), rice cannot synthesize dammarane-type ginsenosides.
METHODS AND RESULTS:
In this study, the ginseng (Panax ginseng CA Mey.) DS gene (GenBank: AB265170.1) was transformed into rice using agrobacterium, and 64 rice transgenic plants were produced. The Transfer-DNA (T-DNA) insertion sites in homozygous lines of the T2 generation were determined by using high-efficiency thermal asymmetric interlaced PCR (hiTAIL-PCR) and differed in all tested lines. One to two copies of the T-DNA were present in each transformant, and real-time PCR and Western blotting showed that the transformed DS gene could be transcribed and highly expressed. High performance liquid chromatography (HPLC) analysis showed that the dammarane-type sapogenin 20(S)-protopanaxadiol (PPD) content was 0.35-0.59 mg/g dw and the dammarane-type sapogenin 20(S)-protopanaxatriol (PPT) content was 0.23-0.43 mg/g dw in the transgenic rice. LC/MS analysis confirmed production of PPD and PPT.
CONCLUSIONS:
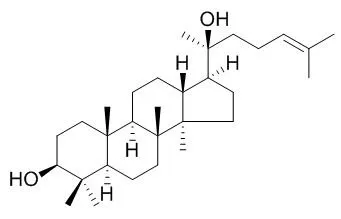
These results indicate that a new "ginseng rice" germplasm containing dammarane-type sapogenins has been successfully developed by transforming the ginseng DS gene into rice. | | Acta Crystallogr Sect E Struct Rep Online. 2012 Nov 1;68(Pt 11):o3089-90. | | 3-epi-Dammarenediol II 1.075 hydrate: a dammarane triterpene from the bark of Aglaia eximia.[Pubmed: 23284420] |
METHODS AND RESULTS:
The title dammarane tritepene, 3α,20(S)-dihy-droxy-dammar-24-ene, which crystallized out in a hydrated form, C(30)H(52)O(2).1.075H(2)O, was isolated from the Aglaia eximia bark. The three cyclo-hexane rings adopt chair conformations. The cyclo-pentane has an envelope conformation with the quaternary C at position 14 as the flap atom with the maximum deviation of 0.288 (2) Å. The methyl-heptene side chain is disordered over two positions with 0.505 (1):0.495 (1) site occupancies and is axially attached with an (+)-syn-clinal conformation. The hydroxyl group at position 3 of dammarane is in a different conformation to the corresponding hydroxyl in Dammarenediol II.
CONCLUSIONS:
In the crystal, the dammarane and water mol-ecules are linked by O(Dammarane)-H⋯O(water) and O(water)-H⋯O(Dammarane) hydrogen bonds into a three-dimensional network. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)