| Structure Identification: |
| Fitoterapia. 2013 Jul;88:38-43. | | Alkyl and phenolic glycosides from Saussurea stella.[Pubmed: 23567860] |
METHODS AND RESULTS:
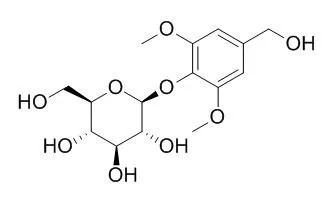
Their structures were elucidated by NMR, MS, UV, and IR spectroscopic analysis. Of the known compounds, (+)-medioresinol-di-O-β-D-glucoside (7), picraquassioside C (10), and diosmetin-3'-O-β-D-glucoside (27) were isolated from the Asteraceae family for the first time, while (+)-pinoresinol-di-O-β-D-glucoside (6), Di-O-methylcrenatin (11), protocatechuic acid (14), 1,5-di-O-caffeoylquinic acid (17), formononetin (28), and phenethyl glucoside (29) were isolated from the Saussurea genus for the first time. The anti-inflammatory activities of three new compounds (1-3), five lignans ((-)-arctiin (4), (+)-pinoresinol-4-O-β-D-glucoside (5), (+)-pinoresinol-di-O-β-D-glucoside (6), (+)-medioresinol-di-O-β-D-glucoside (7) and (+)-syringaresinol-4-O-β-D-glucoside (8)), one neolignan (picraquassioside C (10)), and one phenolic glycoside (Di-O-methylcrenatin (11)) were evaluated by testing their inhibition of the release of β-glucuronidase from PAF-stimulated neutrophils.
CONCLUSIONS:
Only compound 5 showed moderate inhibition of the release of β-glucuronidase, with an inhibition ratio of 39.1%. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)