| In vitro: |
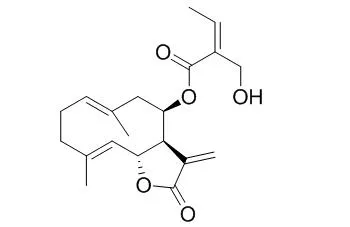
| Chem Pharm Bull (Tokyo). 2002 Sep;50(9):1250-4. | | Seven germacranolides, eupaglehnins A, B, C, D, E, and F, and 2alpha-acetoxyepitulipinolide from Eupatorium glehni.[Reference: WebLink] | We have been interested in biologically active terpenoids from Compositae9—14) and collected E. glehni6—8) in Tokushima and Hokkaido, Japan.
METHODS AND RESULTS:
From the ethyl acetate-soluble fraction of the methanol extract, we have found four new germacranolides with unsaturated esters at the 8-position, eupaglehnin A, eupaglehnin B, Eupaglehnin C, and eupaglehnin D, and two new chlorine atom-containing germacranolides, eupaglehnin E (5) and eupaglehnin F (6),13) as well as 2a-acetoxyepitulipinolide (7) for the first time from natural sources. In our attempt to find cytotoxic compounds, eupatoriopicrin (8) 24—29) was the most effective (1.40 mg/ml), followed by Eupaglehnin C (3) (2.19 mg/ml) against HeLa-S3. However, eupaglehnin E (5) and eupaglehnin F (6) did not show cytotoxic activity, which is understandable because they had no exomethylene group.
CONCLUSIONS:
In preliminary experiments, eupatoriopicrin (8) 24—26) also showed apoptotic activity against Ha-60 cell lines, the details of which will be published in due course. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)