| In vitro: |
| Curr Med Chem. 2014;21(20):2322-30. | | In vitro, in vivo and in silico analysis of the anticancer and estrogen-like activity of guava leaf extracts.[Pubmed: 24438525] | Anticancer drug research based on natural compounds enabled the discovery of many drugs currently used in cancer therapy.
METHODS AND RESULTS:
Here, we report the in vitro, in vivo and in silico anticancer and estrogen-like activity of Psidium guajava L. (guava) extracts and enriched mixture containing the meroterpenes Guajadial, psidial A and psiguadial A and B. All samples were evaluated in vitro for anticancer activity against nine human cancer lines: K562 (leukemia), MCF7 (breast), NCI/ADR-RES (resistant ovarian cancer), NCI-H460 (lung), UACC-62 (melanoma), PC-3 (prostate), HT-29 (colon), OVCAR-3 (ovarian) and 786-0 (kidney). Psidium guajava's active compounds displayed similar physicochemical properties to estradiol and tamoxifen, as in silico molecular docking studies demonstrated that they fit into the estrogen receptors (ERs). The meroterpene-enriched fraction was also evaluated in vivo in a Solid Ehrlich murine breast adenocarcinoma model, and showed to be highly effective in inhibiting tumor growth, also demonstrating uterus increase in comparison to negative controls.
CONCLUSIONS:
The ability of Guajadial, psidial A and psiguadials A and B to reduce tumor growth and stimulate uterus proliferation, as well as their in silico docking similarity to tamoxifen, suggest that these compounds may act as Selective Estrogen Receptors Modulators (SERMs), therefore holding significant potential for anticancer therapy. | | Journal of Hunan University of Chinese Medicine, 2014 , 34 (8) :17-20.CFN97539 | | Sesquiterpenoids with α-Glucosidase Inhibitory Activities from the Leaves of Psidium guajava Linn[Reference: WebLink] | To study the α-glucosidase inhibitory active compounds from the ethyl acetate extract of guava leaves.
METHODS AND RESULTS:
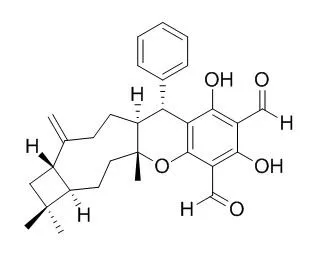
The compounds were separated from the ethyl acetate extract by repeating silica gel and Sephadex LH-20 column chromatography. The 1H-NMR,13C-NMR techniques were used to identify the chemical structures. The α-glucosidase inhibitory activities were tested by colorimetry. Three special meroterpenoids were isolated and identified as psidials A, psiguadial A and Guajadial. Guajadial showed α-glucosidase inhibitory activities significantly and its activity was better than that of Acarbose.
CONCLUSIONS:
Guajadial as one of the α-glucosidase inhibitory active compound from Guava leaves was reported for the first time. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)