| Kinase Assay: |
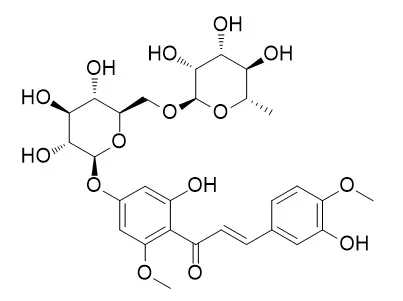
| Int Immunopharmacol. 2008 May;8(5):670-8. | | Hesperidin, hesperidin methyl chalone and phellopterin from Poncirus trifoliata (Rutaceae) differentially regulate the expression of adhesion molecules in tumor necrosis factor-alpha-stimulated human umbilical vein endothelial cells.[Pubmed: 18387509] | The fruits of Poncirus trifoliata (L.) are widely used in Oriental medicine to treat allergic inflammation. Recently, several active compounds including hesperidin,
Hesperidin methylchalcone and phellopterin from P. trifoliata (Rutaceae) were isolated and characterized.
The goal of this study was to investigate the differential effect of hesperidin, hesperidin methylchalone and phellopterin derived from P. trifoliata (Rutaceae) on the induction of intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) by TNF-alpha and the possible molecular mechanisms by which they differentially regulate ICAM-1 and VCAM-1 expressions.
METHODS AND RESULTS:
Stimulation of human umbilical vein endothelial cells (HUVECs) with TNF-alpha resulted in the increase of ICAM-1 and VCAM-1 expressions, while pretreatment with the three components completely inhibited VCAM-1 expression in a dose-dependent manner but had no effect on ICAM-1 expression. All three compounds failed to block TNF-alpha-induced phosphorylation of ERK1/2, which is involved in regulating ICAM-1 production by TNF-alpha. Furthermore, they efficiently inhibited the phosphorylation of Akt and PKC, suggesting that Akt or PKC pathways are an important target by which these compounds regulate TNF-alpha-induced VCAM-1 but not ICAM-1. Additionally, treatment with these chemicals also inhibited U937 monocyte adhesion to HUVECs stimulated with TNF-alpha. Interestingly, the inhibitory effect of hesperidin,
Hesperidin methylchalcone and phellopterin on monocyte adhesion to HUVECs was recapitulated by transfecting cells with VCAM-1 siRNA.
CONCLUSIONS:
Taken together, hesperidin, Hesperidin methylchalcone and phellopterin reduce TNF-alpha-induced VCAM-1 expression through regulation of the Akt and PKC pathway, which contributes to inhibit the adhesion of monocytes to endothelium. |
|
| Animal Research: |
| J Agric Food Chem. 2018 Jun 27;66(25):6269-6280. | | Hesperidin Methylchalcone Suppresses Experimental Gout Arthritis in Mice by Inhibiting NF-κB Activation.[Pubmed: 29852732 ] |
Gout arthritis is a painful inflammatory disease induced by monosodium urate (MSU) crystals. We evaluate the therapeutic potential of the flavonoid Hesperidin methylchalcone (HMC) in a mouse model of gout arthritis induced by intra-articular injection of MSU (100 μg/10 μL).
METHODS AND RESULTS:
Orally given HMC (3-30 mg/kg, 100 μL) reduced in a dose-dependent manner the MSU-induced hyperalgesia (44%, p < 0.05), edema (54%, p < 0.05), and leukocyte infiltration (70%, p < 0.05). HMC (30 mg/kg) inhibited MSU-induced infiltration of LysM-eGFP+ cells (81%, p < 0.05), synovitis (76%, p < 0.05), and oxidative stress (increased GSH, FRAP, and ABTS by 62, 78, and 73%, respectively; reduced O2- and NO by 89 and 48%, p < 0.05) and modulated cytokine production (reduced IL-1β, TNF-α, IL-6, and IL-10 by 35, 72, 37, and 46%, respectively, and increased TGF-β by 90%, p < 0.05). HMC also inhibited MSU-induced NF-κB activation (41%, p < 0.05), gp91phox (66%, p < 0.05) and NLRP3 inflammasome components mRNA expression in vivo (72, 77, 71, and 73% for NLRP3, ASC, pro-caspase-1, and pro-IL-1 β, respectively, p < 0.05), and induced Nrf2/HO-1 mRNA expression (3.9- and 5.1-fold increase, respectively, p < 0.05). HMC (30, 100, and 300 μM) did not inhibit IL-1β secretion by macrophages primed by LPS and challenged with MSU (450 μg/mL), demonstrating that the anti-inflammatory effect of HMC in gout arthritis depends on inhibiting NF-κB but not on direct inhibition of inflammasome.
CONCLUSIONS:
The pharmacological effects of HMC indicate its therapeutic potential for the treatment of gout. | | J Photochem Photobiol B. 2015 Jul;148:145-153. | | Hesperidin methyl chalcone inhibits oxidative stress and inflammation in a mouse model of ultraviolet B irradiation-induced skin damage.[Pubmed: 25916506 ] | Hesperidin methylchalcone (HMC) is a safe flavonoid used to treat chronic venous diseases, but its effects and mechanisms on UVB irradiation-induced inflammation and oxidative stress have never been described in vivo. Thus, the purpose of this study was to evaluate the effects of systemic administration of HMC in skin oxidative stress and inflammation induced by UVB irradiation.
METHODS AND RESULTS:
To induce skin damage, hairless mice were exposed to an acute UVB irradiation dose of 4.14 J/cm(2), and the dorsal skin samples were collected to evaluate oxidative stress and inflammatory response. The intraperitoneal treatment with HMC at the dose of 300 mg/kg inhibited UVB irradiation-induced skin edema, neutrophil recruitment, and matrix metalloproteinase-9 activity. HMC also protected the skin from UVB irradiation-induced oxidative stress by maintaining ferric reducing antioxidant power (FRAP), 2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) radical (ABTS) scavenging ability and antioxidant levels (reduced glutathione and catalase). Corroborating, HMC inhibited UVB irradiation-induced superoxide anion generation and gp91phox (NADPH oxidase subunit) mRNA expression. Furthermore, the antioxidant effect of HMC resulted in lower production of inflammatory mediators, including lipid hydroperoxides and a wide range of cytokines.
CONCLUSIONS:
Taken together, these results unveil a novel applicability of HMC in the treatment of UVB irradiation-induced skin inflammation and oxidative stress. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)