Isocorynoxeine, one of the major alkaloids from Uncaria Hook, shows the effects of lowering blood pressure, vasodilatation, and protection against ischemia-induced neuronal damage.
METHODS AND RESULTS:
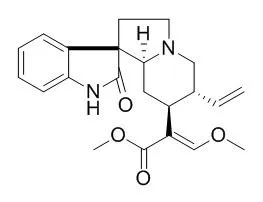
In this paper, the metabolism of Isocorynoxeine was investigated in rats. Twelve metabolites and the parent drug were isolated by using solvent extraction and repeated chromatographic methods, and determined by spectroscopic methods including UV, MS, NMR, and CD experiments. Seven new compounds were identified as 11-hydroxyIsocorynoxeine, 5-oxoisocorynoxeinic acid-22-O-β-D-glucuronide, 10-hydroxyIsocorynoxeine, 17-O-demethyl-16,17-dihydro-5-oxoIsocorynoxeine, 5-oxoisocorynoxeinic acid, 21-hydroxy-5-oxoIsocorynoxeine, and oxireno[18, 19]-5-oxoIsocorynoxeine, together with six known compounds identified as Isocorynoxeine, 18,19-dehydrocorynoxinic acid, 18,19-dehydrocorynoxinic acid B, corynoxeine, Isocorynoxeine-N-oxide, and corynoxeine-N-oxide. Possible metabolic pathways of Isocorynoxeine are proposed.
Furthermore, the activity assay for the parent drug and some of its metabolites showed that Isocorynoxeine exhibited a significant neuroprotective effect against glutamate-induced HT22 cell death at the maximum concentration.
However, little or weak neuroprotective activities were observed for M-3, M-6, M-7, and M-10.
CONCLUSIONS:
Our present study is important to further understand their metabolic fate and disposition in humans. |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)