| In vitro: |
| J Endocrinol. 2002 Nov;175(2):289-96. | | The phytochemical lindleyin, isolated from Rhei rhizoma, mediates hormonal effects through estrogen receptors.[Pubmed: 12429027] | Some plant compounds or herb mixtures are popular alternatives to conventional therapies and contain organic compounds that bind to some nuclear receptors, such as the estrogen receptor (ER), to exert various biological effects. We studied the effect of various herbal extracts on ERalpha and ERbeta isoforms.
METHODS AND RESULTS:
One herbal extract, Rhei rhizoma (rhubarb), acts as an agonist to both ERalpha and ERbeta. The phytochemical Lindleyin, a major component of rhubarb, might contribute to this estrogenic activity through ERalpha and ERbeta. 4-Hydroxytamoxifen, an ER antagonist, completely reversed the estrogenic activity of Lindleyin. Lindleyin binds to ERalpha in vitro, as demonstrated using a fluorescent polarization assay. The in vivo effect of rhubarb extract was studied using a vitellogenin assay system in the freshwater fish, Japanese medaka (Oryzias latipes). There were marked increases in serum vitellogenin levels in male medaka exposed to rhubarb extract.
CONCLUSIONS:
We conclude that Lindleyin, a component of some herbal medicines, is a novel phytoestrogen and might trigger many of the biological responses evoked by the physiological estrogens. | | Chem Pharm Bull (Tokyo). 2000 Jul;48(7):1055-61. | | Prolyl endopeptidase inhibitory activity of fourteen Kampo formulas and inhibitory constituents of Tokaku-joki-to.[Pubmed: 10923840] | Prolyl endopeptidase (PEP, EC 3.4.21.26) is an enzyme playing a role in the metabolism of proline-containing neuropeptides which have been suggested to be involved in learning and memory processes.
METHODS AND RESULTS:
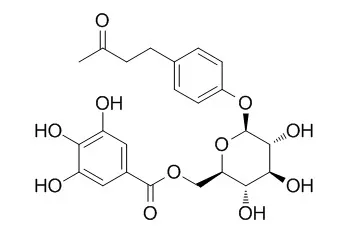
In screening for PEP inhibitors from fourteen traditional Kampo formulas, we found that Tokaku-joki-to shows a significant inhibitory activity. Examination of the constituents of the Kampo formula resulted in the isolation of two new compounds, cis-3,5,4'-trihydroxystilbene 4'-O-beta-D-(6-O-galloyl)glucopyranoside (10) and 4-(4-hydroxyphenyl)-2-butanone 4'-O-beta-D-(6-O-galloyl-2-O-cinnamoyl)glucopyranoside (16), along with twenty-five known compounds, cinnamic acid (1), protocatechuic acid (2), gallic acid (3), torachrysone 8-O-beta-D-glucoside (4), emodin (5), emodin 8-O-beta-D-glucoside (6), 3,5,4'-trihydroxystilbene 4'-O-beta-D-glucopyranoside (7), 3,5,4'-trihydroxystilbene 4'-O-beta-D-(2-O-galloyl)glucopyranoside (8), 3,5,4'-trihydroxystilbene 4'-O-beta-D-(6-O-galloyl)glucopyranoside (9), 4-(4-hydroxyphenyl)-2-butanone 4'-O-beta-D-glucopyranoside (11), isoLindleyin (12), Lindleyin (13), 4(4-hydroxyphenyl)-2-butanone 4'-O-beta-D-(2,6-di-O-galloyl)glucopyranoside (14), 4-(4-hydroxyphenyl)-2-butanone 4'-O-beta-D-(2-O-galloyl-6-O-cinnamoyl)glucopyranoside (15), 1-O-galloylglucose (17), 1,2,6-tri-O-galloylglucose (18), gallic acid 4-O-beta-D-(6-O-galloyl)glucopyranoside (19), liquiritigenin (20), liquiritigenin 4'-O-beta-D-glucoside (21), liquiritigenin 7,4'-diglucoside (22), liquiritigenin 4'-O-beta-D-apiofuranosyl-(1-->2)-beta-D-glucopyranoside (23), licuroside (24), (-)-epicatechin (25), (-)-epicatechin 3-O-gallate (26) and (+)-catechin (27).
CONCLUSIONS:
Among these compounds, twelve (7-10, 14-16, 18, 19, 24-26) showed noncompetitive inhibition with an IC50 of 22.9, 3.0, 14.9, 2.8, 10.5, 0.69, 8.2, 0.44, 9.39, 26.5, 28.1 and 0.052 microM, respectively. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)