| In vitro: |
| Phytochemistry. 2014 Oct;106:61-8. | | Phytotoxic flavonoids from roots of Stellera chamaejasme L. (Thymelaeaceae).[Pubmed: 25096753 ] | Allelopathy, the negative effect on plants of chemicals released to the surroundings by a neighboring plant, is an important factor which contributes to the spread of some weeds in plant communities.
METHODS AND RESULTS:
In this field, Stellera chamaejasme L. (Thymelaeaceae) is one of the most toxic and ecologically-threatening weeds in some of the grasslands of north and west China. Bioassay-guided fractionation of root extracts of this plant led to the isolation of eight flavonoids 1-8, whose structures were elucidated by spectroscopic analysis. All compounds obtained, except 7-methoxylneochaejasmin A (4) and (+)-epiafzelechin (5), showed strong phytotoxic activity against Arabidopsis thaliana seedlings. Seedling growth was reduced by neochamaejasmin B (Neochamaejasmine B,1), mesoneochamaejasmin A (2), chamaejasmenin C (3), genkwanol A (6), daphnodorin B (7) and dihydrodaphnodorin B (8) with IC50 values of 6.9, 12.1, 43.2, 74.8, 7.1 and 27.3ug/mL, respectively, and all of these compounds disrupted root development.
CONCLUSIONS:
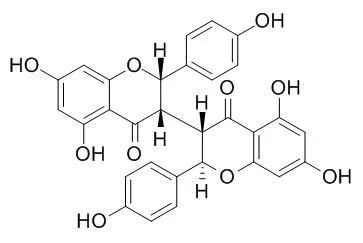
Endogenous auxin levels at the root tips of the A. thaliana DR5::GUS transgenic line were largely reduced by compounds 1, 2 and 6-8, and were increased by compound 4. Moreover, the inhibition rate of A. thaliana auxin transport mutants pin2 and aux1-7 by compounds 1-8 were all lower than the wild type (Col-0). | | Natural Product Research & Development, 2014 , 26 (5) :639-44. | | Nematicidal Activities of Isoneochamaejasmin A and Neochamaejasmin B from the Roots of Stellera chamaejasme L.against Bursaphelenchus xylophilus and Bursaphelenchus mucronatus[Reference: WebLink] | Two biflavonoids were isolated from ethanol extract from the roots of Stellera chamaejasme L.,and their chemical structures were identified as isoneochamaejasmin A and neochamaejasmin B(Neochamaejasmine B) by nuclear magnetic resonance spectral data.
METHODS AND RESULTS:
The nematicidal activities of the two compounds were tested by bioassay methods with second-stage juveniles(J2s)of Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. The results showed these two biflavonoids exhibited significantly nematicidal activities against both B. xylophilus and B. mucronatus. Neochamaejasmin B (Neochamaejasmine B)displayed better nematicidal activity than isoneochamaejasmin A against two species nematodes under the same conditions. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)