| Structure Identification: |
| J Nat Prod. 2015 Nov 25;78(11):2565-71. | | Flavoalkaloids and Flavonoids from Astragalus monspessulanus.[Pubmed: 26558405 ] |
METHODS AND RESULTS:
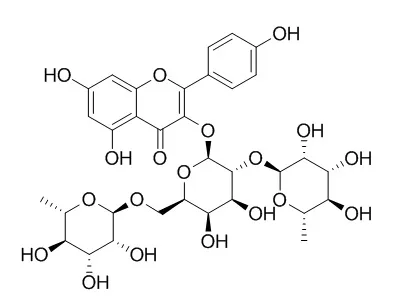
A new flavonol tetraglycoside, quercetin-3-O-[α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-galactopyranosyl]-7-O-β-D-glucopyranoside (1), and two new flavonol alkaloids, N-(8-methylquercetin-3-O-[α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-galactopyranosyl])-3-hydroxypiperidin-2-one (2) and N-(8-methylkaempferol-3-O-[α-L-rhamnopyranosyl-(1→2)-[α-L-rhamnopyranosyl-(1→6)]-β-D-galactopyranosyl])-3-hydroxypiperidin-2-one (3), were isolated from the aerial parts of Astragalus monspessulanus ssp. monspessulanus.
Two rare flavonoids with an unusual 3-hydroxy-3-methylglutaric acid moiety, quercetin-3-O-α-L-rhamnopyranosyl-(1→2)-[6-O-(3-hydroxy-3-methylglutaryl)-β-D-galactopyranoside (4) and kaempferol-3-O-α-L-rhamnopyranosyl-(1→2)-[6-O-(3-hydroxy-3-methylglutaryl)-β-D-galactopyranoside (5), were isolated from the aerial parts of A. monspessulanus ssp. illyricus. In addition, the eight known flavonoids alangiflavoside (6), alcesefoliside (7), Mauritianin (8), quercetin-3-β-robinobioside (9), cosmosine (10), apigenin-4'-O-glucoside (11), trifolin (12), and rutin (13) were isolated from aerial parts of A. monspessulanus ssp. monspessulanus.
CONCLUSIONS:
Their structures were elucidated via NMR and HRESIMS data. In a model that tested t-BuOOH-induced oxidative stress on isolated rat hepatocytes, flavonoids 1-13 had statistically significant cytoprotective activity similar to that of silymarin, tested at 60 μg/mL. The most prominent effects were observed for flavonoids 1, 4, 7, and 12. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)