| Structure Identification: |
| Carbohydr Res. 2009 Sep 8;344(13):1770-4. | | Glycosylated nervogenic acid derivatives from Liparis condylobulbon (Reichb.f.) leaves.[Pubmed: 19664759] |
METHODS AND RESULTS:
Three new Nervogenic acid glycosides, 1-O-alpha-L-rhamnopyranosyl 3,5-bis(3-methyl-but-2-enyl)-4-O-[alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranosyl]-benzoate, 3,5-bis(3-methyl-but-2-enyl)-4-O-[alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranosyl]-benzoic acid, and bis{3,5-bis(3-methyl-but-2-enyl)-4-O-[alpha-L-rhamnopyranosyl-(1-->2)-beta-D-glucopyranosyl]-benzoyl} 1,2-O-beta-d-glucopyranose, which we named condobulbosides A-C, were isolated from a methanol extract of the leaves of Liparis condylobulbon together with an apigenin C-glycoside, schaftoside.
CONCLUSIONS:
Their structures were established on the basis of spectral techniques, namely, UV, IR, HR-MS spectroscopy, both 1D and 2D NMR experiments, and chemical reactions. | | Planta Med. 1993 Dec;59(6):546-51. | | Five new prenylated p-hydroxybenzoic acid derivatives with antimicrobial and molluscicidal activity from Piper aduncum leaves.[Pubmed: 8302955 ] |
METHODS AND RESULTS:
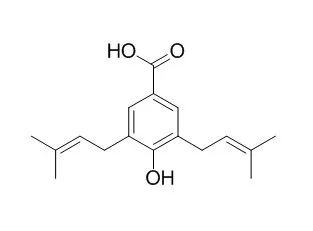
Five new prenylated benzoic acid derivatives, methyl 3-(3,7-dimethyl-2,6-octadienyl)-4-methoxybenzoate (1), 1-(1-methylethyl)-4-methyl-3-cyclohexenyl 3,5-bis(3-methyl-2-butenyl)-4-hydroxybenzoate (2), 1-(1-methylethyl)-4-methyl-3-cyclohexenyl 3,5-bis(3-methyl-2-butenyl)-4-methoxybenzoate (3), methyl 3,5-bis(3-methyl-2-butenyl)-4-methoxybenzoate (4), and 4-hydroxy-3-(3-methyl-2-butenyl)-5-(3-methyl-2-butenyl)-benzoic acid (5) were isolated from the dried leaves of Piper aduncum L. (Piperaceae). Together with the new metabolites, four known prenylated benzoic acid derivatives, 3,5-bis(3-methyl-2-butenyl)-4-methoxybenzoic acid (6), 4-hydroxy-3,5-bis(3-methyl-2-butenyl)-benzoic acid (Nervogenic acid, 7), methyl 4-hydroxy-3,5-bis(3-methyl-2-butenyl)-benzoate (8), and methyl 4-hydroxy-3-(3-methyl-2-butenyl)-benzoate (9) as well as, dillapiol (10), myristicin, and the three sesquiterpenes humulene, caryophyllene epoxide, and humulene epoxide were isolated. Compounds 7, 8, and 9 are reported as natural products for the first time. The structures of the isolates were elucidated by spectroscopic methods, mainly 1D-and 2D-NMR spectroscopy.
CONCLUSIONS:
Isolates 4-7, 9, and 10 were molluscicidal while 2, 5-7, and 9 displayed significant antibacterial activities. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)