| In vitro: |
| Phytochemistry, 1973 , 12 (7) :1657-67. | | Furanocoumarin biosynthesis in Ruta graveolens cell cultures[Reference: WebLink] | The biosynthetic routes to four linear furanocoumarins—psoralen, xanthotoxin, bergapten. isopimpinellin-co-occurring in Ruta graveolens cell cultures have been investigated with six 14C-labelled compounds.
METHODS AND RESULTS:
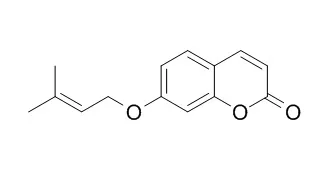
Mevalonic acid was only poorly incorporated, in contrast to umbelliferone. In support of previous suggestions, 7-demethylsuberosin and (±)-marmesin were very good precursors of the linear furanocoumarins. 7-O-Prenylumbelliferone(7-Prenylumbelliferone) also was fairly well utilized, but this was probably owing to a prior ether cleavage yielding umbelliferone. Psoralen was well incorporated into bergapten and xanthotoxin, but not into the dimethoxylated isopimpinellin. Differences exist between the organized plant and its cell culture in terms of metabolic products and, by implication, precursor utilization. S(+)-Marmesin was obtained in small quantity from an acid-hydrolysable conjugate present in the culture medium.
CONCLUSIONS:
Syntheses of [2-14C]7-demethylsuberosin, [2-14C]osthenol, [2-14C]7-O-prenylumbelliferone, [3-14C] (±)-marmesin, and [3-14C]psoralen are described, as well as an improved method for separation of furanocoumarin mixtures by TLC and GLC. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)