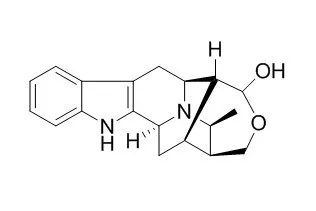
| Structure Identification: |
| J Org Chem. 2014 Nov 7;79(21):10030-48. | | General strategy for synthesis of C-19 methyl-substituted sarpagine/macroline/ajmaline indole alkaloids including total synthesis of 19(S),20(R)-dihydroperaksine, 19(S),20(R)-dihydroperaksine-17-al, and peraksine.[Pubmed: 25247616] |
METHODS AND RESULTS:
A detailed account of the development of a general strategy for synthesis of the C-19 methyl-substituted alkaloids including total synthesis of 19(S),20(R)-dihydroPeraksine-17-al (1), 19(S),20(R)-dihydroPeraksine (2), and Peraksine (6) is presented. Efforts directed toward the total synthesis of macrosalhine chloride (5) are also reported. Important to success is the sequence of chemical reactions which include a critical haloboration reaction, regioselective hydroboration, and controlled oxidation (to provide sensitive enolizable aldehydes at C-20). In addition, the all-important Pd-catalyzed α-vinylation reaction has been extended to a chiral C-19 alkyl-substituted substrate for the first time.
CONCLUSIONS:
Synthesis of the advanced intermediate 64 completes an improved formal total synthesis of talcarpine (26) and provides a starting point for synthesis of macroline-related alkaloids 27-31.
Similarly, extension of this synthetic strategy in the ring A oxygenated series should provide easy access to the northern hemisphere 32b of the bisindoles angustricraline, alstocraline, and foliacraline (Figure 4 ). | | J Ethnopharmacol. 1984 Jun;11(1):99-117. | | Stem bark alkaloids of Rauvolfia caffra.[Pubmed: 6471882] |
METHODS AND RESULTS:
Thirty two alkaloids were isolated from the stem bark of Rauvolfia caffra and 28 were identified. The alkaloids represented corynane (3), strictamine (1), sarpagan (4), akuammicine (2), pleiocarpamine (1), indolenine (1), dihydroindole (6), Peraksine (3), heteroyohimbine (2), hydroxyheteroyohimbine (2), oxindole (1), 2-acyl-indole (1), suaveoline (3) and yohimbine (2) types. The anhydronium base serpentine was detected but not isolated.
CONCLUSIONS:
The principal alkaloids were ajmaline and norajmaline (dihydroindoles), ajmalicinine and ajmalicine (heteroyohimbines), geissoschizol (E-seco indole) and pleiocarpamine and the heteroyohimbine derived alkaloids were predominantly normal configuration compounds.
The biosynthetic and ethnopharmacological significance of the alkaloids is discussed. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)