| In vitro: |
| Molecules. 2015 Jun 25;20(7):11719-32. | | Transport of Twelve Coumarins from Angelicae Pubescentis Radix across a MDCK-pHaMDR Cell Monolayer-An in Vitro Model for Blood-Brain Barrier Permeability.[Pubmed: 26121397] | Angelicae Pubescentis Radix (APR), a widely used traditional Chinese medicine, is reported to have central nervous system activities.
METHODS AND RESULTS:
The purpose of this study was to characterize the blood-brain barrier permeability of twelve coumarins from APR including umbelliferone (1), osthol (2), scopoletin (3), Peucedanol (4), ulopterol (5), angepubebisin (6), psoralen (7), xanthotoxin (8), bergapten (9), isoimperatorin (10), columbianadin (11), and columbianetin acetate (12) with an in vitro model using a MDCK-pHaMDR cell monolayer. The cell monolayer was validated to be suitable for the permeation experiments. The samples' transports were analyzed by high performance liquid chromatography and their apparent permeability coefficients (Papp) were calculated. According to the Papp value, most coumarins could be characterized as well-absorbed compounds except for 4, 10 and 11 which were moderately absorbed ones, in concentration-dependent and time-dependent manners. The results of P-glycoprotein (P-gp) inhibitor (verapamil) experiments showed that the transport of coumarin 4 was affected by the transport protein P-gp. Sigmoid functions between permeability log(Papp AP-BL*MW0.5) and log D (at pH 7.4) were established to analyze the structure-activity relationship of coumarins.
CONCLUSIONS:
The results provide useful information for discovering the substance basis for the central nervous system activities of APR, and predicting the permeability of other coumarins through BBB. | | J Agric Food Chem. 2003 Aug 27;51(18):5255-61. | | Antioxidant compounds from the leaves of Peucedanum japonicum thunb.[Pubmed: 12926867] |
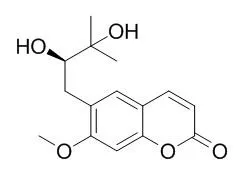
Seventeen compounds were isolated from the n-butanol soluble fraction of the leaves of Peucedanum japonicum Thunb.
METHODS AND RESULTS:
On the basis of MS and various NMR spectroscopic techniques, the structures of the isolated compounds were determined as isoquercitrin (1), rutin (2), 3-O-caffeoylquinic acid (3), 4-O-caffeoylquinic acid (4), 5-O-caffeoylquinic acid (5), cnidioside A (6), praeroside II (7), praeroside III (8), apterin (9), esculin (10), (R)-Peucedanol (11), (R)-Peucedanol 7-O-beta-d-glucopyranoside (12), l-tryptophan (13), uracil (14), guanosine (15), uridine (16), and thymidine (17). All compounds except 11 and 12 were isolated for the first time from P. japonicum. Several isolated compounds were quantified by high-performance liquid chromatography analysis. In addition, all isolated compounds were examined for radical scavenging on 1,1-diphenyl-2-picrylhydrazyl radical and for inhibition of oxidation of liposome induced by 2,2'-azobis(2-amidinopropane)dihydrochloride.
CONCLUSIONS:
Compounds 2-5 were found to be the major potent constituents, which contribute to the antioxidant activity of P. japonicum leaves. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)