| In vivo: |
| Eur J Pharmacol. 1998 May 29;350(1):101-8. | | Opioid activity of alkaloids extracted from Picralima nitida (fam. Apocynaceae).[Pubmed: 9683021] | Extracts of the seeds of Picralima nitida (fam. Apocynaceae) have been reported to have opioid analgesic activity.
METHODS AND RESULTS:
In this investigation, isolated tissue bioassays and radioligand binding assays have been used to determine the opioid activity of five alkaloids--akuammidine, akuammine, akuammicine, akuammigine and Pseudoakuammigine--extracted from the seeds of P. nitida. Akuammidine showed a preference for mu-opioid binding sites with Ki values of 0.6, 2.4 and 8.6 microM at mu-, delta- and kappa-opioid binding sites, respectively. The agonist actions of akuammidine in the mouse-isolated vas deferens were antagonised by naloxone and the mu-opioid receptor selective antagonist D-Phe-Cys-Tyr-D-Trp-Orn-Thr-Pen-Thr-NH2 (CTOP) confirming an action at mu-opioid receptors. In contrast, akuammine also showed highest affinity for mu-opioid binding sites (Ki 0.5 microM) but was an antagonist at mu-opioid receptors with a pK(B) of 5.7 against the selective mu-opioid receptor agonist [D-Ala2,MePhe4,Gly-ol5]enkephalin (DAMGO). Akuammicine has the highest affinity for kappa-opioid binding sites (Ki 0.2 microM) and was a full agonist at kappa-opioid receptors in the guinea pig ileum preparation but a partial kappa-opioid receptor agonist in the vasa deferentia of the mouse and the rabbit. Akuammigine and Pseudoakuammigine showed little or no efficacy in the opioid bioassays. None of the alkaloids had significant activity for opioid receptor-like binding sites (ORL1-binding sites) with Ki values >> 10 microM.
CONCLUSIONS:
These data show that some alkaloids extracted from the medicinal plant P. nitida possess varying degrees of agonist and antagonist activity at opioid receptors but possess neither high affinity nor selectivity for mu-, delta- or kappa-opioid receptors or the ORL1-receptor. | | Curr Med Chem. 2003 Sep;10(18):1891-915. | | Current progress in the chemistry and pharmacology of akuammiline alkaloids.[Pubmed: 12871110] |
METHODS AND RESULTS:
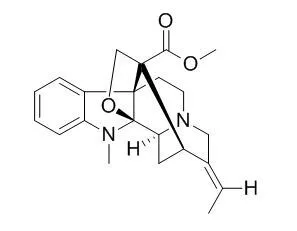
Akuammiline alkaloids are a family of monoterpene indole alkaloids of renewed medicinal interest.
These bases act as ligands for a heterogeneous group of molecular targets and, consequently, display a wide variety of pharmacological activities. For example, Pseudoakuammigine (2) exhibits opioid activity in vivo, echitamine (4) has been reported to have promising cytotoxic activity, and corymine (121) behaves as an antagonist of the glycine receptor. Oddly enough, these alkaloids have not raised enough interest in the organic synthesis community, remaining inaccessible; even the entry to their pentacyclic framework continues elusive. Recently, several akuammiline bases have been isolated and identified including bisindole alkaloids, such as vingramine (103) or rausutrine (110), which incorporate akuammiline-type subunits.
CONCLUSIONS:
This review covers the advances in the chemistry and pharmacology of akuammiline alkaloids reported within the last ten years. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)