| In vitro: |
| Planta Med. 2010 Oct;76(15):1654-8. | | Raspberry ketone increases both lipolysis and fatty acid oxidation in 3T3-L1 adipocytes.[Pubmed: 20425690 ] | Raspberry ketone (RK) is a natural phenolic compound of the red raspberry. The dietary administration of RK to male mice has been reported to prevent high-fat diet-induced elevation in body weight and to increase lipolysis in white adipocytes.
METHODS AND RESULTS:
To elucidate a possible mechanism for the antiobesity action of RK, its effects on the expression and the secretion of adiponectin, lipolysis, and fatty acid oxidation in 3T3-L1 were investigated. Treatment with 10 μM of RK increased lipolysis significantly in differentiated 3T3-L1 cells. An immunoassay showed that RK increased both the expression and the secretion of adiponectin, an adipocytokine mainly expressed and secreted by adipose tissue. In addition, treatment with 10 μM of RK increased the fatty acid oxidation and suppressed lipid accumulation in 3T3-L1 adipocytes.
CONCLUSIONS:
These findings suggest that RK holds great promise as an herbal medicine since its biological activities alter the lipid metabolism in 3T3-L1 adipocytes. | | J Med Food. 2014 Mar;17(3):332-8. | | Raspberry ketone promotes the differentiation of C3H10T1/2 stem cells into osteoblasts.[Pubmed: 24404978 ] | The decrease in the bone mass associated with osteoporosis caused by ovariectomy, aging, and other conditions is accompanied by an increase in bone marrow adipose tissue. The balance between osteoblasts and adipocytes is influenced by a reciprocal relationship. The development of modalities to promote local/systemic bone formation by inhibiting bone marrow adipose tissue is important in the treatment of fractures or metabolic bone diseases such as osteoporosis.
METHODS AND RESULTS:
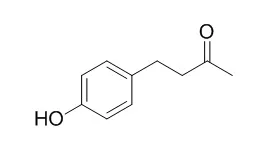
In this study, we examined whether Raspberry ketone [4-(4-hydroxyphenyl)butan-2-one; RK], which is one of the major aromatic compounds of red raspberry and exhibits anti-obesity action, could promote osteoblast differentiation in C3H10T1/2 stem cells. Confluent C3H10T1/2 stem cells were treated for 6 days with 10-100 μg/mL of RK in culture medium containing 10 nM all-trans-retinoic acid (ATRA) or 300 ng/mL recombinant human bone morphogenetic protein (rhBMP)-2 protein as an osteoblast-differentiating agent. RK in the presence of ATRA increased alkaline phosphatase (ALP) activity in a dose-dependent manner. RK in the presence of rhBMP-2 also increased ALP activity. RK in the presence of ATRA also increased the levels of mRNAs of osteocalcin, α1(I) collagen, and TGF-βs (TGF-β1, TGF-β2, and TGF-β3) compared with ATRA only. RK promoted the differentiation of C3H10T1/2 stem cells into osteoblasts. However, RK did not affect the inhibition of early-stage adipocyte differentiation.
CONCLUSIONS:
Our results suggest that RK enhances the differentiation of C3H10T1/2 stem cells into osteoblasts, and it may promote bone formation by an action unrelated to adipocyte differentiation. | | Journal of Dermatological Science, 2016, 84(1):e178-e178. | | Different effects of five depigmentary compounds, rhododendrol, raspberry ketone, monobenzone, rucinol and AP736 on viability of melanocytes[Reference: WebLink] | Numerous medications are used to treat hyperpigmentation. However, several reports have indicated that repeated application of some agents, such as rhododendrol (RD), Raspberry ketone (RK) and monobenzone (MB), can be toxic to melanocytes. Although these agents had severe side effects in human trials, no current in vitro methods can predict the safety of such drugs.
METHODS AND RESULTS:
This study assessed the in vitro effects of five depigmentary compounds including leukoderma-inducing agents. In particular, we determined the effects of different concentrations and exposure times of different depigmentary agents on cell viability and melanogenesis in the presence and absence of ultraviolet B (UVB) radiation. Concentrations of RD, RK and MB that inhibit melanogenesis are similar to concentrations that are cytotoxic; however, concentrations of rucinol (RC) and AP736 that inhibit melanogenesis are much lower than concentrations that are cytotoxic. Furthermore, the concentrations that cause toxic effects depend on exposure duration, and prolonged exposure to RD, RK and MB had more cytotoxic effects than prolonged exposure to RC and AP736. The cytotoxic effects of RD and RK appear to be mediated by apoptosis due to increased expression of caspase-3 and caspase-8; UVB radiation increased the cytotoxicity of these agents and also increased caspase activity.
CONCLUSIONS:
Our results indicate that different leukoderma-inducing compounds have different effects on the viability of normal epidermal melanocytes and suggest that the in vitro assay used here can be used to predict whether an investigational compound that induces leukoderma may lead to adverse effects in human trials. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)