| In vitro: |
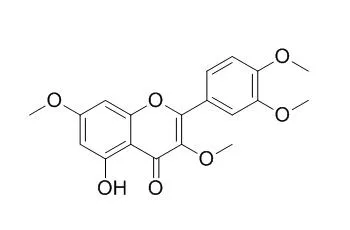
| J Agric Food Chem. 2015 Sep 23;63(37):8106-15. | | Flavonoids Affect the Light Reaction of Photosynthesis in Vitro and in Vivo as Well as the Growth of Plants.[Pubmed: 26322527 ] | Flavonoids Retusin (5-hydroxy-3,7,3',4'-tetramethoxyflavone) (1) and pachypodol (5,4'-dihydroxy-3,7,3'-trimethoxyflavone) (2) were isolated from Croton ciliatoglanduliferus Ort.
METHODS AND RESULTS:
Pachypodol acts as a Hill reaction inhibitor with its target on the water splitting enzyme located in PSII. In the search for new herbicides from natural compounds, flavonoids 1 and 2 and flavonoid analogues quercetin (3), apigenin (4), genistein (5), and eupatorin (6) were assessed for their effect in vitro on the photosynthetic electron transport chain and in vivo on the germination and growth of the plants Physalis ixocarpa, Trifolium alexandrinum and Lolium perenne. Flavonoid 3 was the most active inhibitor of the photosynthetic uncoupled electron flow (I50 = 114 μM) with a lower log P value (1.37).
CONCLUSIONS:
Results in vivo suggest that 1, 2, 3, and 5 behave as pre- and postemergent herbicides, with 3 and 5 being more active. | | Chem Biodivers. 2015 Jun;12(6):963-79. | | Free-Radical-Scavenging, Antityrosinase, and Cellular Melanogenesis Inhibitory Activities of Synthetic Isoflavones.[Pubmed: 26080742] | In this study, we examined the potential of synthetic isoflavones for application in cosmeceuticals.
METHODS AND RESULTS:
Twenty-five isoflavones were synthesized and their capacities of free-radical-scavenging and mushroom tyrosinase inhibition, as well as their impact on cell viability of B16F10 murine melanoma cells and HaCaT human keratinocytes were evaluated. Isoflavones that showed significant mushroom tyrosinase inhibitory activities were further studied on reduction of cellular melanin formation and antityrosinase activities in B16F10 melanocytes in vitro. Among the isoflavones tested, 6-hydroxydaidzein (2) was the strongest scavenger of both ABTS(.+) and DPPH(.) radicals with SC50 values of 11.3 ± 0.3 and 9.4 ± 0.1 μM, respectively. Texasin (20) exhibited the most potent inhibition of mushroom tyrosinase (IC50 14.9 ± 4.5 μM), whereas Retusin (17) showed the most efficient inhibition both of cellular melanin formation and antityrosinase activity in B16F10 melanocytes, respectively.
CONCLUSIONS:
In summary, both Retusin (17) and texasin (20) exhibited potent free-radical-scavenging capacities as well as efficient inhibition of cellular melanogenesis, suggesting that they are valuable hit compounds with potential for advanced cosmeceutical development. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)