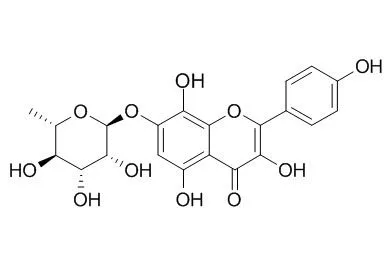
Isolation of compounds from the root of Rhodiola sachalinensis (RRS) yielded tyrosol (1), salidroside (2), multiflorin B (3), kaempferol-3,4'-di-O-β-D-glucopyranoside (4), afzelin (5), kaempferol (6), Rhodionin (7), and rhodiosin (8).
METHODS AND RESULTS:
Quantification of these compounds was performed by high-performance liquid chromatography (HPLC). To investigate the antioxidant and anti-inflammatory effects of the compounds, DPPH radical scavenging, NBT superoxide scavenging and nitric oxide production inhibitory activities were examined in LPS-stimulated Raw 264.7 cells. We suggest that the major active components of RRS are herbacetin glycosides, exhibiting antioxidant activity, and kaempferol, exhibiting anti-inflammatory activity.In this study, 1,1-diphenyl-2-picryl-hydrazyl (DPPH) radical scavenging activity and nitrotetrazolium blue chloride (NBT) superoxide scavenging activity were measured to assess the antioxidant activity of the components from RRS. DPPH has the ability to easily accept hydrogen atoms because it contains an unstable element, the hydrazyl nitrogen, therefore, antioxidant activity can be measured because DPPH loses its violet color when it receives hydrogens from antioxidants [18]. Additionally, NBT has the ability to easily receive superoxide because it contains unstable anions. Therefore, antioxidant activity may be measured when NBT loses its yellow color upon reaction with abundant superoxide [19]. Among the compounds from RRS, 7(Rhodionin) and 8 exhibited the most potent DPPH free radical scavenging activities, with IC50 values of 19.49 ± 0.21 and 27.77 ± 0.61 μM, respectively, compared to the positive control, L-ascorbic acid (IC50 = 32.89 ± 0.70 μM). The other compounds did not exhibit activities in this assay up to 100 μM (Table 2). |





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)