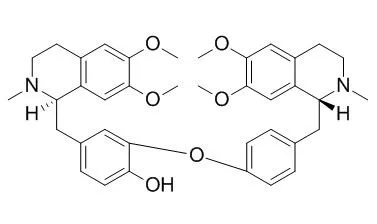
| Description: |
Dauricine may has neuroprotective, and anti-tumor effects, it can pass the blood‑brain barrier, and that P‑glycoprotein has an important role in the transportation of Dauricine across the blood‑brain barrier, it can inhibit tumor cells in urinary system and colon cancer cell proliferation, invasion; induce cell apoptosis by suppressing NF-kappaB activity and the expression profile of its downstream genes. Dauricine also has pulmonary toxicity, can produce pulmonary injury in CD-1 mice by the metabolism of Dauricine mediated by CYP3A. |
| In vitro: |
| Asian Pac J Trop Med. 2012 Dec;5(12):973-6. | | Dauricine can inhibit the activity of proliferation of urinary tract tumor cells.[Pubmed: 23199717] | To explore the anti-tumor effects of asiatic moonseed rhizome extraction-Dauricine on bladder cancer EJ cell strain, prostate cancer PC-3Mcell strain and primary cell culture system.
METHODS AND RESULTS:
The main effective component-phenolic alkaloids ofMenispermum dauricum was extracted and separated from asiatic moonseed rhizome by chemical method. MTT method was used to detect Dauricine anti-tumor effect.
Dauricine had an obvious proliferation inhibition effect on the main tumor cells in urinary system. The minimum drug sensitivity concentration was between 3.81-5.15 μg/mL, and the inhibition ratio increased with the increase of concentration.
CONCLUSIONS:
Dauricine, the main effective component extracted from asiatic moonseed rhizome, had a good inhibition effect on tumor cells in urinary system. At the same time, Dauricine has certain inhibition effects on the primary cultured tumor cell. | | J Cell Physiol. 2010 Oct;225(1):266-75. | | Dauricine induces apoptosis, inhibits proliferation and invasion through inhibiting NF-kappaB signaling pathway in colon cancer cells.[Pubmed: 20509140] | Dauricine, a bioactive component of Asiatic Moonseed Rhizome, has been widely used to treat a large number of inflammatory diseases in traditional Chinese medicine.
METHODS AND RESULTS:
In our study, we demonstrated that Dauricine inhibited colon cancer cell proliferation and invasion, and induced apoptosis by suppressing nuclear factor-kappaB (NF-kappaB) activation in a dose- and time-dependent manner. Addition of Dauricine inhibited the phosphorylation and degradation of IkappaBalpha, and the phosphorylation and translocation of p65. Moreover, Dauricine down-regulated the expression of various NF-kappaB-regulated genes, including genes involved cell proliferation (cyclinD1, COX2, and c-Myc), anti-apoptosis (survivin, Bcl-2, XIAP, and IAP1), invasion (MMP-9 and ICAM-1), and angiogenesis (VEGF). In athymic nu/nu mouse model, we further demonstrated that Dauricine significantly suppressed colonic tumor growth.
CONCLUSIONS:
Taken together, our results demonstrated that Dauricine inhibited colon cancer cell proliferation, invasion, and induced cell apoptosis by suppressing NF-kappaB activity and the expression profile of its downstream genes. These findings provide evidence for a novel role of Dauricine in preventing or treating colon cancer through modulation of NF-kappaB singling pathway. |
|
| In vivo: |
| Mol Med Rep. 2014 Mar;9(3):985-8. | | P‑glycoprotein inhibition increases the transport of dauricine across the blood‑brain barrier.[Pubmed: 24378368] | Dauricine is the major bioactive component isolated from the roots of Menispermum dauricum D.C. The aim of the present study was to investigate the role of P‑glycoprotein in the transport of Dauricine across the blood‑brain barrier by pre‑treatment with the P‑glycoprotein inhibitor verapamil.
METHODS AND RESULTS:
Sprague Dawley rats were divided into a verapamil group (pretreated with verapamil at a dose of 20 mg/kg) and a control group (pretreated with the same volume of normal saline). After 90 min, the animals were injected intravenously with Dauricine (10 mg/kg). At 15, 30 and 60 min after Dauricine administration, the levels of Dauricine in the blood and brain were detected by high‑performance liquid chromatography. Compared with the control group, the Dauricine concentration in the brains of the rats in the verapamil group was significantly increased. Furthermore, the brain‑plasma ratio of Dauricine in the rats pretreated with verapamil was significantly higher than that of the animals in the control group. However, there was no difference identified between Dauricine levels in the plasma of the verapamil and the control groups.
CONCLUSIONS:
The results indicated that Dauricine is able to pass the blood‑brain barrier, and that P‑glycoprotein has an important role in the transportation of Dauricine across the blood‑brain barrier. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)