| Kinase Assay: |
| Int J Cancer. 2012 Aug 15;131(4):1003-8. | | Rocaglamide and a XIAP inhibitor cooperatively sensitize TRAIL-mediated apoptosis in Hodgkin's lymphomas.[Pubmed: 21952919] | Although most of the patients with Hodgkin's lymphoma (HL) can be cured by the current regimen of high-dose multiagent chemotherapy, the treatment causes high risks of later toxicities including secondary malignancies.
Therefore, new rational strategies are needed for HL treatment. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a promising anticancer agent due to its tumor selectivity and its lack of toxicity for normal cells. Unfortunately, many cancers remain resistant to TRAIL including HL. HL is characterized by enhanced expression of cellular caspase-8 (FLICE)-inhibitory protein (c-FLIP) and X-linked inhibitor of apoptosis (XIAP), which block receptor-mediated apoptosis by inhibiting caspase-8 and caspase-3, respectively.
METHODS AND RESULTS:
We have recently discovered the herbal compound Rocaglamide, which breaks TRAIL-resistance in acute T cell leukemia through inhibition of c-FLIP expression. We have also shown that small molecule XIAP inhibitors can sensitize TRAIL-mediated apoptosis in several resistant tumors. However, whether targeting XIAP or c-FLIP is also a suitable strategy to prime HL cells for TRAIL-induced apoptosis has not yet been investigated. In our study, we show that Rocaglamide suppresses c-FLIP expression in HL cells in a dose- and time-dependent manner. However, downregulation of c-FLIP alone was not sufficient to sensitize TRAIL-induced apoptosis in HL cells. Similarly, treatment of HL cells with a small molecule XIAP inhibitor resulted in a moderate induction of apoptosis. However, inhibition of XIAP alone was also not sufficient to enhance TRAIL-induced cell death. Synergistic increase in TRAIL-mediated killing of HL cells was only obtained by combination of Rocaglamide and XIAP inhibitors.
CONCLUSIONS:
Our study demonstrates that targeting both c-FLIP and XIAP are necessary for an efficient treatment of HL. | | J Biol Chem. 2002 Nov 22;277(47):44791-800. | | Rocaglamide derivatives are potent inhibitors of NF-kappa B activation in T-cells.[Pubmed: 12237314 ] | Crude extracts from different Aglaia species are used as anti-inflammatory remedies in the traditional medicine of several countries from Southeast Asia.
Because NF-kappaB transcription factors represent key regulators of genes involved in immune and inflammatory responses, we supposed that the anti-inflammatory effects of Aglaia extracts are mediated by the inhibition of NF-kappaB activity.
METHODS AND RESULTS:
Purified compounds of Aglaia species, namely 1H-cyclopenta[b]benzofuran lignans of the Rocaglamide type as well as one aglain congener were tested for their ability to inhibit NF-kappaB activity. We show that a group of Rocaglamides represent highly potent and specific inhibitors of tumor necrosis factor-alpha (TNFalpha) and phorbol 12-myristate 13-acetate (PMA)-induced NF-kappaB-dependent reporter gene activity in Jurkat T cells with IC(50) values in the nanomolar range. Some derivatives are less effective, and others are completely inactive. Rocaglamides are able to suppress the PMA-induced expression of NF-kappaB target genes and sensitize leukemic T cells to apoptosis induced by TNFalpha, cisplatin, and gamma-irradiation. The suppression of NF-kappaB activation correlated with the inhibition of induced IkappaB(alpha) degradation and IkappaB(alpha) kinase activation. The level of interference was determined and found to be localized upstream of the IkappaB kinase complex but downstream of the TNF receptor-associated protein 2.
CONCLUSIONS:
Our data suggest that Rocaglamide derivatives could serve as lead structures in the development of anti-inflammatory and tumoricidal drugs. | | Cell Death Dis. 2014 Jan 16;5:e1000. | | The traditional Chinese medical compound Rocaglamide protects nonmalignant primary cells from DNA damage-induced toxicity by inhibition of p53 expression.[Pubmed: 24434508] | Targeting the cancer cell cycle machinery is an important strategy for cancer treatment. Cdc25A is an essential regulator of cycle progression and checkpoint response. Over-expression of Cdc25A occurs often in human cancers.
METHODS AND RESULTS:
In this study, we show that Rocaglamide-A (Roc-A), a natural anticancer compound isolated from the medicinal plant Aglaia, induces a rapid phosphorylation of Cdc25A and its subsequent degradation and, thereby, blocks cell cycle progression of tumor cells at the G1-S phase. Roc-A has previously been shown to inhibit tumor proliferation by blocking protein synthesis. In this study, we demonstrate that besides the translation inhibition Roc-A can induce a rapid degradation of Cdc25A by activation of the ATM/ATR-Chk1/Chk2 checkpoint pathway. However, Roc-A has no influence on cell cycle progression in proliferating normal T lymphocytes. Investigation of the molecular basis of tumor selectivity of Roc-A by a time-resolved microarray analysis of leukemic vs. proliferating normal T lymphocytes revealed that Roc-A activates different sets of genes in tumor cells compared with normal cells. In particular, Roc-A selectively stimulates a set of genes responsive to DNA replication stress in leukemic but not in normal T lymphocytes.
CONCLUSIONS:
These findings further support the development of Rocaglamide for antitumor therapy. |
|


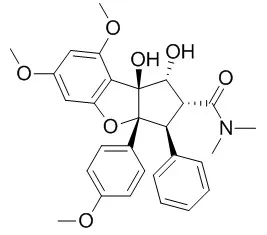



 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)