| In vitro: |
| Microb Cell Fact. 2016 Aug 4;15(1):134. | | Engineering Saccharomyces cerevisiae with the deletion of endogenous glucosidases for the production of flavonoid glucosides.[Pubmed: 27491546 ] | Glycosylation of flavonoids is a promising approach to improve the pharmacokinetic properties and biological activities of flavonoids. Recently, many efforts such as enzymatic biocatalysis and the engineered Escherichia coli biotransformation have increased the production of flavonoid glucosides. However, the low yield of flavonoid glucosides can not meet the increasing demand for human medical and dietary needs. Saccharomyces cerevisiae is a generally regarded as safe (GRAS) organism that has several attractive characteristics as a metabolic engineering platform for the production of flavonoid glucosides. However, endogenous glucosidases of S. cerevisiae as a whole-cell biocatalyst reversibly hydrolyse the glucosidic bond and hinder the biosynthesis of the desired products. In this study, a model flavonoid, scutellarein, was used to exploit how to enhance the production of flavonoid glucosides in the engineered S. cerevisiae.
METHODS AND RESULTS:
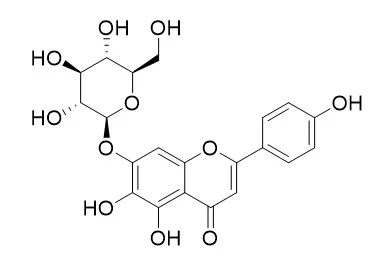
To produce flavonoid glucosides, three flavonoid glucosyltransferases (SbGTs) from Scutellaria baicalensis Georgi were successfully expressed in E. coli, and their biochemical characterizations were identified. In addition, to synthesize the flavonoid glucosides in whole-cell S. cerevisiae, SbGT34 was selected for constructing the engineering yeast. Three glucosidase genes (EXG1, SPR1, YIR007W) were knocked out using homologous integration, and the EXG1 gene was determined to be the decisive gene of S. cerevisiae in the process of hydrolysing flavonoid glucosides. To further enhance the potential glycosylation activity of S. cerevisiae, two genes encoding phosphoglucomutase and UTP-glucose-1-phosphate uridylyltransferase involved in the synthetic system of uridine diphosphate glucose were over-expressed in S. cerevisiae. Consequently, approximately 4.8 g (1.2 g/L) of Scutellarein-7-O-glucoside (S7G) was produced in 4 L of medium after 54 h of incubation in a 10-L fermenter while being supplied with ~3.5 g of scutellarein.
CONCLUSIONS:
The engineered yeast harbouring SbGT with a deletion of glucosidases produced more flavonoid glucosides than strains without a deletion of glucosidases. This platform without glucosidase activity could be used to modify a wide range of valued plant secondary metabolites and to explore of their biological functions using whole-cell S. cerevisiae as a biocatalyst. | | Phytother Res. 2014 Sep;28(9):1399-405. | | The influence of selected flavonoids from the leaves of Cirsium palustre (L.) Scop. on collagen expression in human skin fibroblasts.[Pubmed: 24643916 ] | Ten flavonoids belonging to the subclasses of flavones, flavanones and aurones were isolated from methanolic extract of Cirsium palustre leaves after multistep chromatographic separation. Their structures were elucidated with spectroscopic methods.
METHODS AND RESULTS:
All compounds, except for luteolin 7-O-glucoside, were isolated for the first time. Four compounds-eriodictyol 7-O-glucoside (6), 6-hydroxyluteolin 7-O-glucoside (11), Scutellarein-7-O-glucoside (12) and pedalitin (14)-were tested for their effect on collagen expression in normal human dermal fibroblasts. Among them, compound 11 at 40 μM and compound 14, at all concentrations used (1, 20, 40 μM), significantly enhanced the level of total collagen secreted into the medium. Furthermore, compound 11 significantly stimulated type I collagen expression, whereas compound 14 activated type I and III collagen expression at the mRNA level, depending on concentration. MMP-2 activity was inhibited by all study compounds, with the greatest effect recorded with compound 14 at 20 μM. The lack of effect on collagen content in the medium of compound 6- and compound 12-treated cells, besides an increase in COL1A1 and COL1A2 expression, might be caused by diminished expression of HSP47 gene, resulting in decreased procollagen secretion.
CONCLUSIONS:
Future study of compounds 11 and 14 for their potential therapeutic use in conditions connected with collagen biosynthesis deficiency is required. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)