| Structure Identification: |
| Tetrahedron.1968;24(8):3247–3253. | | Components of seseli sibiricum: Constitution and synthesis of sibiricin, a new coumarin.[Reference: WebLink] |
METHODS AND RESULTS:
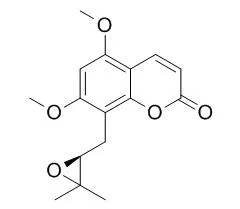
From the pet. ether extract of S. sibiricum osthol, imperatorin, bergapten and a new coumarin named Sibiricin have been isolated. It has been assigned 5,7-dimethoxy-coumarin-8-γ,γ-dimethylallyl epoxide structure on the basis of degradative and spectral studies.
CONCLUSIONS:
Final confirmation of the structure has been provided by a synthesis following an unambigous route using 3-dimethylallyl-2-hydroxy-4,6-dimethoxybenzaldehyde as the important intermediate. | | Chemical & Pharmaceutical Bulletin, 1996 , 44 (6) :1208-1211. | | Prenylcoumarins from Murraya paniculata var. omphalocarpa(Rutaceae): The Absolute Configuration of Sibiricin, Mexoticin and Omphamurin.[Reference: WebLink] |
METHODS AND RESULTS:
Three new prenylcoumarins, murpaniculol senecioate, 5-methoxymurrayatin and omphamurin isovalerate were isolated from the leaves of Murraya paniculata var. omphalocarpa (Rutaceae), together with six known coumarins, paniculatin, (-)-Sibiricin, 5, 7-dimethyoxy-8-(3'-methyl-2'-oxobutyl)coumarin, (-)-mexoticin, omphamurin and omphalocarpin. Their structures were characterizied on the basis of spectroscopic evidence.
CONCLUSIONS:
The absolute configulation of omphamurin at the C-2' position has been determined to be S by Horeau's method. THe absolute stereochemistry of (-)-mexoticin and (-)-Sibiricin has also been established by their chemical correlation with omphamurin. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)