| In vitro: |
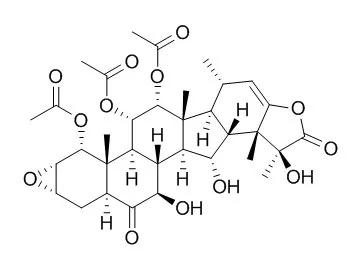
| J. Am. Chem. Soc., 2011, 133 (47), pp 19064–19067 | | Potent Taccalonolides, AF and AJ, Inform Significant Structure–Activity Relationships and Tubulin as the Binding Site of These Microtubule Stabilizers[Reference: WebLink] | The taccalonolides are a class of microtubule stabilizing agents isolated from plants of the genus Tacca. In efforts to define their structure–activity relationships, we isolated five new taccalonolides, AC–AF and H2, from one fraction of an ethanol extract of Tacca plantaginea.
METHODS AND RESULTS:
The structures were elucidated using a combination of spectroscopic methods, including 1D and 2D NMR and HR-ESI-MS. Taccalonolide AJ, an epoxidation product of Taccalonolide B, was generated by semisynthesis. Five of these taccalonolides demonstrated cellular microtubule-stabilizing activities and antiproliferative actions against cancer cells, with taccalonolide AJ exhibiting the highest potency with an IC50 value of 4.2 nM. The range of potencies of these compounds, from 4.2 nM to >50 μM, for the first time provides the opportunity to identify specific structural moieties crucial for potent biological activities as well as those that impede optimal cellular effects. In mechanistic assays, taccalonolides AF and AJ stimulated the polymerization of purified tubulin, an activity that had not previously been observed for taccalonolides A and B, providing the first evidence that this class of microtubule stabilizers can interact directly with tubulin/microtubules. Taccalonolides AF and AJ were able to enhance tubulin polymerization to the same extent as paclitaxel but exhibited a distinct kinetic profile, suggesting a distinct binding mode or the possibility of a new binding site.
CONCLUSIONS:
The potencies of taccalonolides AF and AJ and their direct interaction with tubulin, together with the previous excellent in vivo antitumor activity of this class, reveal the potential of the taccalonolides as new anticancer agents. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)