| Description: |
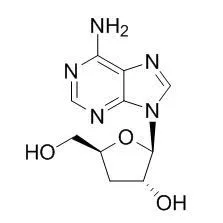
Cordycepin possesses immunological stimulating,anti-hyperglycemia, anti-cancer, neuroprotective, antifungal, antibacterial, anti-inflammatory, anti-virus and anti-infection activities. Cordycepin has inhibitory effects on osteoclast differentiation in vitro and that it suppresses inflammatory bone loss in vivo. Cordycepin inhibited the production of NO production by down-regulation of iNOS and COX-2 gene expression via the suppression of NF-κB activation, Akt and p38 phosphorylation, suppressed HMGA2, Twist1 and ZEB1-dependent melanoma invasion and metastasis by targeting miR-33b. |
| Targets: |
HMG-CoA Reductase | NF-kB | p38MAPK | TGF-β/Smad | MMP(e.g.TIMP) | NO | NOS | Akt | COX | TNF-α | ERK | JNK | ROS | Calcium Channel | Caspase | Antifection |
| In vitro: |
| Eur J Pharmacol. 2006 Sep 18;545(2-3):192-9. | | Cordycepin inhibits lipopolysaccharide-induced inflammation by the suppression of NF-kappaB through Akt and p38 inhibition in RAW 264.7 macrophage cells.[Pubmed: 16899239] | Cordyceps militaris, a caterpillar-grown traditional medicinal mushroom, produces an important bioactive compound, Cordycepin (3'-deoxyadenosine). Cordycepin is reported to possess many pharmacological activities including immunological stimulating, anti-cancer, anti-virus and anti-infection activities. The molecular mechanisms of Cordycepin on pharmacological and biochemical actions of macrophages in inflammation have not been clearly elucidated yet.
METHODS AND RESULTS:
In the present study, we tested the role of Cordycepin on the anti-inflammation cascades in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophage cells. In LPS-activated macrophage, nitric oxide (NO) production was inhibited by butanol fraction of C. militaris and the major component of C. militaris butanol faction was identified as Cordycepin by high performance liquid chromatography. To investigate the mechanism by which Cordycepin inhibits NO production and inducible nitric oxide synthase (iNOS) expression, we examined the activation of Akt and MAP kinases in LPS-activated macrophage. Cordycepin markedly inhibited the phosphorylation of Akt and p38 in dose-dependent manners in LPS-activated macrophage. Moreover, Cordycepin suppressed tumor necrosis factor (TNF-alpha) expression, IkappaB alpha phosphorylation, and translocation of nuclear factor-kappaB (NF-kappaB). The expressions of cycloxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS) were significantly decreased in RAW 264.7 cell by Cordycepin.
CONCLUSIONS:
Taken together, these results suggest that Cordycepin inhibits the production of NO production by down-regulation of iNOS and COX-2 gene expression via the suppression of NF-kappaB activation, Akt and p38 phosphorylation. Thus, Cordycepin may provide a potential therapeutic approach for inflammation-associated disorders. | | J Agric Food Chem. 2000 Jul;48(7):2744-8. | | Cordycepin: selective growth inhibitor derived from liquid culture of Cordyceps militaris against Clostridium spp.[Pubmed: 10898616] | The growth responses of nine human intestinal bacteria to liquid culture of Cordyceps militaris Link. Pt. (Ascomycotina: Clavicipitaceae) collected from a pupa of Bombyx mori L. (Lepidoptera: Bombycidae) were examined using spectrophotometric and impregnated paper disk methods and compared to those of tetracycline and chloramphenicol, as well as those of Coptis japonica root-derived berberine chloride.
METHODS AND RESULTS:
The biologically active constituent of the cultures was characterized as Cordycepin (3'-deoxyadenosine) by spectroscopic analysis. This compound revealed potent growth-inhibiting activity toward Clostridium paraputrificum and Clostridium perfringens at 10 microgram/disk without adverse effects on the growth of Bifidobacterium bifidum, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium adolescentis, Lactobacillus acidophilus, and Lactobacillus casei, whereas tetracycline and chloramphenicol inhibited the growth of these lactic acid-producing bacteria, clostridia and Escherichia coli. However, C. militaris-derived materials revealed no growth stimulation on the bifidobacteria and lactobacilli.
CONCLUSIONS:
These results may be an indication of at least one of the pharmacological actions of C. militaris. As a naturally occurring antibacterial agent, Cordycepin could be useful as a new preventive agent against various diseases caused by clostridia. | | PLoS One . 2019 Jun 14;14(6):e0218449. | | Cordycepin kills Mycobacterium tuberculosis through hijacking the bacterial adenosine kinase[Pubmed: 31199855] | | Abstract
Cordycepin is an efficient component of Cordyceps spp, a traditional Chinese medicine widely used for healthcare in China, and has been recently acted as a strong anticancer agent for clinic. However, whether and how it may play a role in combating tuberculosis, caused by Mycobacterium tuberculosis, remains unknown. Here we report that Cordycepin can kill Mycobacterium by hijacking the bacterial adenosine kinase (AdoK), a purine salvage enzyme responsible for the phosphorylation of adenosine (Ado) to adenosine monophosphate (AMP). We show that Cordycepin is a poor AdoK substrate but it competitively inhibits the catalytic activity of AdoK for adenosine phosphorylation. Cordycepin does not affect the activity of the human adenosine kinase (hAdoK), whereas hAdoK phosphorylates Cordycepin to produce a new monophosphate derivative. Co-use of Cordycepin and deoxycoformycin, an inhibitor of adenosine deaminase (ADD), more efficiently kills M. bovis and M. tuberculosis. The add-deleted mycobacterium is more sensitive to Cordycepin. This study characterized Cordycepin as a new mycobactericidal compound and also uncovered a potential anti-mycobacterial mechanism. |
|
| In vivo: |
| Nutr Res. 2015 May;35(5):431-9. | | Cordycepin from Cordyceps militaris prevents hyperglycemia in alloxan-induced diabetic mice.[Pubmed: 25940982] | Cordyceps militaris has long been used in prescriptions of traditional Chinese medicine as a tonic for the treatment of metabolic syndrome. Cordycepin with proven immunomodulatory, antitumor, and hepatoprotective properties is the main active metabolite of C militaris.
Diabetes mellitus is a group of metabolic diseases in which the body is unable to regulate blood sugar levels. Hence, we hypothesized that Cordycepin can normalize blood sugar levels and improve the indicators of diabetes.
METHODS AND RESULTS:
The aim of this study was to investigate the possible effects of Cordycepin from C militaris on diabetes in an alloxan-induced diabetic mouse model. Diabetic mice were intraperitoneally administered different doses of Cordycepin (8, 24, and 72 mg/kg body weight) daily for 21 days. Acute toxicity test on normal mice was carried out by giving them maximum tolerance dose of Cordycepin (3600 mg/kg) daily. A 47% reduction of the blood glucose level, 214% increase of hepatic glycogen content, and significant improvement of oral glucose tolerance were noticed after the effective dose of Cordycepin was administered. Polyphagia and polydipsia, the typical symptoms of diabetes, were partly alleviated. Moreover, Cordycepin offered protective effects against diabetes-related kidney and spleen injury. Maximum tolerance dose test indicated that Cordycepin at the large dose of 3600 mg/kg did not show significant effect on body weight and major organ in normal mice after intraperitoneal administration for 14 days.
CONCLUSIONS:
The results showed that Cordycepin from C militaris that elicited hypoglycemic activity contributes to the regulation of glucose metabolism in liver in alloxan-induced diabetic mice. Therefore, a Cordycepin treatment during diabetes can improve some of the metabolic syndrome symptoms by regulation of glucose absorption in vivo. Cordycepin may serve as a therapeutic agent in the treatment of diabetes and its related complications. | | Zhongguo Zhong Yao Za Zhi. 2014 Nov;39(21):4096-101. | | Mechanisms of cordycepin on improving renal interstitial fibrosis via regulating eIF2α/TGF-β/Smad signaling pathway[Pubmed: 25775775] |
To investigate the effects and mechanisms of Cordycepin,an effective component of cordyceps militaris, on renal interstitial fibrosis (RIF) and its related eIF2α/TGF-β/Smad signaling pathway.
METHODS AND RESULTS:
Firstly, 15 C57BL/6 mice were randomly divided into 3 groups,the control group (Group A), the model group (Group B) and the Cordycepin-treated group (Group C). After renal interstitial fibrotic model was successfully established by unilateral ureteral obstruction (UUO), the mice in Group C were intraperitoneally administrated with Cordycepin(5 mg x kg(-1) d(-1)) and the ones in Group A and B were administrated with physiological saline for 5 days. At the end of the study, the obstructed kidneys were collected and detected for the pathological changes of RIF, and the mRNA expressions of collagen type I (Col I) and α-smooth muscle actin (α-SMA) in the kidney by Northern blot. Secondly, after renal tubular epithelial (NRK-52E) cells cultured in vitro were exposed to transforming growth factor (TGF) -β with or without Cordycepin, the mRNA expressions of Col I and collagen type IV( Col IV) by Northern blot, and the protein expressions of eukaryotic initiation factor 2α (eIF2α), phosphorylated eIF2α ( p-eIF2α), Smad2/3 and phosphorylated Smad2/3 (p-Smad2/3) were tested by Western blot.
In vivo, Cordycepin alleviated RIF in model mice, including improving fibrotic pathological characteristics and mRNA expressions of Col I and α-SMA. In vitro, Cordycepin induced the high expression of p-elF2α, and inhibited the expressions of p-Smad2/3, Col I and Col IV induced by TGF-β in NRK-52E cells.
CONCLUSIONS:
Cordycepin attenuates RIF in vivo and in vitro, probably by inducing the phosphorylation of eIF2α, suppressing the expression of p-Smad2/3, a key signaling molecule in TGF-β/Smad signaling pathway, and reducing the expressions of collagens and α-SMA in the kidney. | | Eur J Pharmacol. 2011 Aug 16;664(1-3):20-8. | | Cordycepin protects against cerebral ischemia/reperfusion injury in vivo and in vitro.[Pubmed: 21554870 ] | Cordycepin, (3'-deoxyadenosine), a bioactive compound of Cordyceps militaris, has been shown to exhibit many pharmacological actions, such as anti-inflammatory, antioxidative and anticancer activities. Little is known about the neuroprotective action of Cordycepin as well as its molecular mechanisms.
METHODS AND RESULTS:
In this study, Cordycepin was investigated for its neuroprotective potential in mice with ischemia following 15 min of the bilateral common carotid artery occlusion and 4h of reperfusion. The effect of Cordycepin was also studied in mice brain slices treated with oxygen-glucose deprivation (OGD) injury. Our results showed that Cordycepin was able to prevent postischemic neuronal degeneration and brain slice injury. Excitatory amino acids such as glutamate and aspartate in brain homogenized supernatant, which were increased in ischemia/reperfusion group, were detected by high performance liquid chromatography (HPLC). The results showed that Cordycepin was able to decrease the extracellular level of glutamate and aspartate significantly. Moreover, Cordycepin was able to increase the activity of superoxide dismutase (SOD) and decrease the level of malondialdehyde (MDA), ameliorating the extent of oxidation. Furthermore, matrix metalloproteinase-3(MMP-3), a key enzyme involved in inflammatory reactions, was markedly increased after ischemia reperfusion, whereas Cordycepin was able to inhibit its expression obviously.
CONCLUSIONS:
In conclusion, our in vivo and in vitro study showed that Cordycepin was able to exert a potent neuroprotective function after cerebral ischemia/reperfusion. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)