| Structure Identification: |
| Magnetic Resonance in Chemistry, 2003, 41(9):731-734. | | 1H and 13C NMR assignments of abietane diterpenes from Aegiphila lhotzkyana.[Reference: WebLink] |
METHODS AND RESULTS:
An NMR study of one new and several known abietane diterpenes isolated from the roots of Aegiphila lhotzkyana is described. In addition to 1D NMR, several 2D shift-correlated NMR pulse sequences (1H–1H-COSY, NOESY, HMQC and HMBC) were used to establish all the structures, and unambiguously perform the 1H and 13C chemical shift assignments of the new natural diterpene and three derivatives, the NMR data for which have not been reported previously. Revision of current data assignment for Teuvincenone H is also suggested. | | Phytochemistry, 1992, 31(5):1697-1701. | | Rearranged abietane diterpenoids from the root of two Teucrium species.[Reference: WebLink] |
METHODS AND RESULTS:
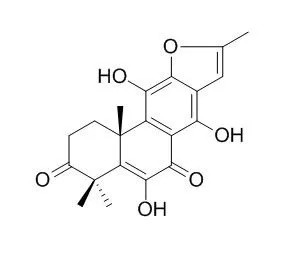
From the root of Teucrium fruticans, two new rearranged abietane diterpenoids, teuvincenones F and G, have been isolated together with the known diterpene teuvincenone E. The acetone extract of the root of T. polium subsp. expansum yielded three previously known compounds (ferruginol and teuvincenones A and B) and two new 17(15 → 16)-abeo-abietane derivatives (teuvincenones H and I).
CONCLUSIONS:
The structures of the new diterpenoids [12,16-epoxy-11,14-dihydroxy-17(15 → 16), 18(4 → 3)-diabeo-abieta-3,5,8,11,13,15-hexaene-2,7-dione (teuvincenone F), (16S)-12,16-epoxy-11,14-dihydroxy-17(15 → 16)-abeo-abieta-8,11,13-triene-3,7-dione (teuvincenone G), 12,16-epoxy-6,11,14-trihydroxy-17(15 → 16)-abeo-abieta-5,8,11,13,15-pentaene-3,7-dione (Teuvincenone H) and 12,16-epoxy-6-hydroxy-17(15 → 16)-abeo-abieta-5,8,12,15-tetraene-3,7,11,14-tetraone (teuvincenone I)] were established by spectroscopic means and by comparison with closely related compounds. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)