| In vitro: |
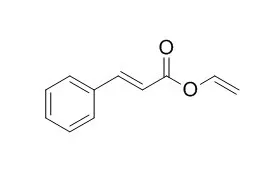
| Macromol Rapid Commun. 2014 Aug;35(15):1345-50. | | Initiated chemical vapor deposition and light-responsive cross-linking of poly(vinyl cinnamate) thin films.[Pubmed: 24817405] | The first vapor-phase deposition of poly(Vinyl Cinnamate) (PVCin) is reported.
METHODS AND RESULTS:
Initiated chemical vapor deposition (iCVD) is used to synthesize Vinyl Cinnamate thin films with an average thickness of 100 nm. Free radical polymerization and cyclization reactions compete during the deposition process, with approximately 45% of the repeat units undergoing cyclization. Exposure to UV light (λ = 254 nm) induces dimerization (cross-linking) of the Vinyl Cinnamate, which is quantified using spectroscopic techniques. Approximately 90% of the free cinnamate moieties are dimerized at a UV dose of 300 mJ cm(-2) . Vinyl Cinnamate is also incorporated into a copolymer with N-isopropylacrylamide, which exhibits a characteristic change in hydrophilicity with temperature.
CONCLUSIONS:
The copolymer is selectively cross-linked through a mask, and reversible swelling of patterns with 30 μm resolution is demonstrated by submerging the film in water. | | Biomacromolecules. 2013 Aug 12;14(8):2937-44. | | Dispersibility and emulsion-stabilizing effect of cellulose nanowhiskers esterified by vinyl acetate and vinyl cinnamate.[Pubmed: 23883187] | The surface of cotton cellulose nanowhiskers (CNW's) was esterified by vinyl acetate (VAc) and Vinyl Cinnamate (VCin), in the presence of potassium carbonate as catalyst. Reactions were performed under microwave activation and monitored by Fourier transform infrared (FT-IR) spectroscopy.
METHODS AND RESULTS:
The supramolecular structure of CNW's before and after modification was characterized by X-ray diffraction (XRD) and atomic force microscopy (AFM). Distinctively from the acetylation treatment, an increase in particles dimensions was noted after esterification with VCin, which was assigned to π-π stacking interactions that may exist between cinnamoyl moieties. The dispersibility and emulsion stabilizing effect of acylated CNW's was examined in ethyl acetate, toluene, and cyclohexane, three organic solvents of medium to low polarity. The acylated nanoparticles could never be dispersed in toluene nor cyclohexane, but they formed stable dispersions in ethyl acetate while remaining dispersible in water.
CONCLUSIONS:
Stable ethyl acetate-in-water, toluene-in-water, and cyclohexane-in-water emulsions were successfully prepared with CNW's grafted with acetyl moieties, whereas the VCin-treated particles could stabilize only the cyclohexane-in-water emulsions. The impact of esterification treatment on emulsion stability and droplets size was particularly discussed. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)