| Description: |
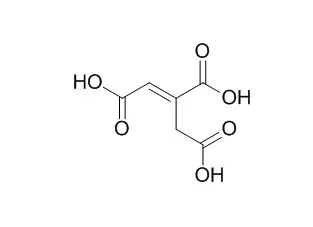
Trans-aconitic acid inhibits the growth and photosynthesis of Glycine max.Trans-aconitic acid has antileishmanial activity ,the diesters of TAA as potential useful derivatives for the management of rheumatoid arthritis and other inflammatory diseases. |
| Targets: |
Antifection |
| In vitro: |
| Plant Physiology & Biochemistry, 2018, 132:490-496. | | Trans-aconitic acid inhibits the growth and photosynthesis of Glycine max.[Reference: WebLink] | Grasses producing trans-Aconitic acid, a geometric isomer of cis-aconitic acid, are often used in Glycine max rotation systems. However, the effects of trans-Aconitic acid on Glycine max are unknown.
METHODS AND RESULTS:
We conducted a hydroponic experiment to evaluate the effects of 2.5–10 mM trans-Aconitic acid on Glycine max growth and photosynthesis. The results revealed that the enhanced H2O2 production in the roots increased the membrane permeability and reduced the water uptake. These effects culminated with a reduced stomatal conductance (gs), which seems to be the main cause for a decreased photosynthetic rate (A). Due to low gs, the limited CO2 assimilation may have overexcited the photosystems, as indicated by the high production of H2O2 in leaves. After 96 h of incubation, and due to H2O2-induced damage to photosystems, a probable non-stomatal limitation for photosynthesis contributed to reducing A. This is corroborated by the significant decrease in the quantum yield of electron flow through photosystem II in vivo (ΦPSII) and the chlorophyll content.
CONCLUSIONS:
Taken together, the damage to the root system and photosynthetic apparatus caused by trans-Aconitic acid significantly reduced the Glycine max plant growth. |
|
| In vivo: |
| Biomedicine & Pharmacotherapy, 2018, 99:87-95. | | Esterification of trans-aconitic acid improves its anti-inflammatory activity in LPS-induced acute arthritis.[Reference: WebLink] | trans-Aconitic acid (TAA) is an abundant constituent in the leaves of Echinodorus grandiflorus, a medicinal plant used to treat rheumatoid arthritis in Brazil. Esterification was explored as a strategy to increase lipophilicity and biopharmaceutical properties of TAA, a highly polar tricarboxylic acid. We herein report the synthesis of TAA esters via Fischer esterification with ethanol, n-butanol and n-octanol.
METHODS AND RESULTS:
The reaction kinetics was investigated to produce mono-, di- and tri- derivatives. Mono- and diesters of TAA were obtained as a mixture of positional isomers, whereas the triesters were recovered as pure compounds. The obtained esters were screened in a model of acute arthritis induced by the injection of LPS in the knee joint of Swiss mice. The diesters were the most active compounds, regardless of the alcohol employed in the reaction, whereas bioactivity of the derivatives improved by increasing the length of the aliphatic chain of the alcohol employed in esterification. In general, the esters showed higher potency than TAA. When administered orally to mice at doses of 0.017-172.3 μmol/Kg, the diethyl, di-n-butyl and di-n-octyl esters of TAA reduced the cellular infiltration into the knee joint, especially of neutrophils.
CONCLUSIONS:
The study identified diesters of TAA as potential useful derivatives for the management of rheumatoid arthritis and other inflammatory diseases. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)