| Structure Identification: |
| Lucia Lopes, Jun 10, 2014 | | Flavonoids, norisoprenoids and other terpenes from leaves of Tapirira guianensis[Reference: WebLink] |
METHODS AND RESULTS:
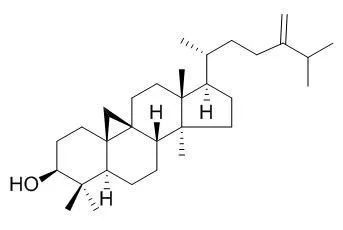
From hexane fraction of methanol extract of leaves of Tapirira guianensis (Anacardiaceae) were obtained lupeol, 24-Methylenecycloartan-3-ol, phytol, α-amyrin, β-amyrin, sitosterol, sitostenone, glycosyl sitosterol, as well as sitosterol esterified with palmitic and stearic acids. Phytol, α-amyrin and β-amyrin esterified with fatty acids were also identified from same extract. The EtOAc extract besides the norisoprenoids (6S,7E,9S)-6,9-dihydroxy-megastigma-4,7-dien -3-one 9-O-β-glucopyranoside and (6S,7E,9R)-6,9-dihydroxy-megastigma-4,7-dien-3-one 9-O-β-glucopyranoside also afforded kaempferol 3-O-rhamnoside, kaempferol 3-O-arabinofuranoside, quercetin 3-O-rhamnoside, and kaempferol.
CONCLUSIONS:
The structural elucidation of isolated compounds were based on UV, IR, MS, 1H and 13C NMR data analysis. | | Rev. Bras Farmacogn., 2008, 18(4): 563-8. | | Chemical constituents from leaves of Murraya paniculata (Rutaceae)[Reference: WebLink] |
METHODS AND RESULTS:
Chromatographic separation of the hexane extract and the CH2Cl2 phase from the ethanol extract from leaves of Murraya paniculata yielded one triterpenoid (24-methylene-cycloartan-3β-ol), one phenylpropanoid (methyl caffeate) and seven coumarins [isomeranzine, murranganone acetate, murrayatine, murrangatine, meranzine hydrate, phebalosine and murranganone].
CONCLUSIONS:
All the isolated coumarins were previously obtained from M. paniculata while 24-methylene-cycloartan-3β-ol(24-Methylenecycloartan-3-ol) and methyl caffeate have been described for first time in the Murraya genus. The crude extracts, fractions and pure substances were submitted to evaluation of antimicrobial potential against Staphylococcus aureus and Escherichia coli which indicated that the coumarin meranzine hydrate showed weak activity. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)