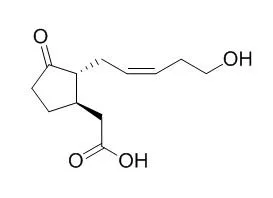
| Structure Identification: |
| J Biol Chem. 2013 Nov 1;288(44):31701-14. | | The amidohydrolases IAR3 and ILL6 contribute to jasmonoyl-isoleucine hormone turnover and generate 12-hydroxyjasmonic acid upon wounding in Arabidopsis leaves.[Pubmed: 24052260] | Jasmonates (JAs) are a class of signaling compounds that mediate complex developmental and adaptative responses in plants.
METHODS AND RESULTS:
JAs derive from jasmonic acid (JA) through various enzymatic modifications, including conjugation to amino acids or oxidation, yielding an array of derivatives. The main hormonal signal, jasmonoyl-L-isoleucine (JA-Ile), has been found recently to undergo catabolic inactivation by cytochrome P450-mediated oxidation. We show that unexpectedly, the abundant accumulation of tuberonic acid (12-Hydroxyjasmonic acid) after wounding originates partly through a sequential pathway involving (i) conjugation of JA to Ile, (ii) oxidation of the JA-Ile conjugate, and (iii) cleavage under the action of the amidohydrolases.
CONCLUSIONS:
The coordinated actions of oxidative and hydrolytic branches in the jasmonate pathway highlight novel mechanisms of JA-Ile hormone turnover and redefine the dynamic metabolic grid of jasmonate conversion in the wound response.
| | J Agric Food Chem. 2006 Jul 26;54(15):5388-92. | | Polar constituents from the aerial parts of Origanum vulgare L. Ssp. hirtum growing wild in Greece.[Pubmed: 16848522] |
METHODS AND RESULTS:
From the polar extracts of Origanum vulgare L. ssp. hirtum 19 compounds have been isolated. The structures and relative stereochemistry have been elucidated by spectroscopic analysis and determined as apigenin, luteolin, chrysoeriol, diosmetin, quercetin, eriodictyol, cosmoside, vicenin-2, caffeic acid, p-menth-3-ene-1,2-diol 1-O-beta-glucopyranoside, thymoquinol 2-O-beta-glucopyranoside, thymoquinol 5-O-beta-glucopyranoside, thymoquinol 2,5-O-beta-diglucopyranoside, 12-Hydroxyjasmonic acid, 12-Hydroxyjasmonic acid 12-O-beta-glucopyranoside, lithospermic acid B, rosmarinic acid, 10-epi-lithospermic acid, and epi-lithospermic acid B.
CONCLUSIONS:
The three latter products display unusual stereochemistry of the 3,4-hydroxyphenyllactic acid unit(s), which to the authors' best knowledge has never been reported before in similar compounds.
Moreover, lithospermic acid B (and its stereoisomers), p-menth-3-ene-1,2-diol 1-O-beta-glucopyranoside, 12-Hydroxyjasmonic acid, and 12-Hydroxyjasmonic acid 12-O-beta-glucopyranoside were isolated for the first time from Origanum species.
|
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)