| Structure Identification: |
| Zhongguo Zhong yao za zhi,2016,41(2):233-249. | | Chemical constituents of Chinese red ginseng.[Pubmed: 28861969] |
METHODS AND RESULTS:
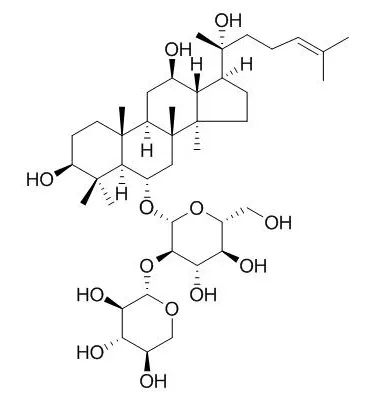
The chemical constituents of the Chinese red ginseng were systematically investigated by using various column chromatographic methods including D-101 macroporous adsorptive resins and open silica gel column chromatographies as well as high-performance liquid chromatography.Their chemical structures were identified by physico-chemical properties and spectral analyses.Fifty-two compounds were isolated from Chinese red ginseng decoction and identified as 20(S)-ginsenoside Rh₁ (1), 20(R)-ginsenoside Rh₁ (2), ginsenoside Rg₆ (3), 20(22) E-ginsenoside F₄ (4), ginsenoside Rk₃ (5), 20(22) E-ginsenoside Rh₄ (6), ginsenoside Rg₁ (7), 20(S)-ginsenoside Rf-1a(8), 20(S)-ginsenoside Rf(9), 20(R)-ginsenoside Rf(10),20(S)-notoginsenoside R₂ (11),20(R)-Notoginsenoside R2 (12), 20(S)-ginsenoside Rg₂ (13), 20(R)-ginsenoside Rg₂ (14), ginsenoside Rs₂ (15),ginsenoside Rs₁ (16),ginsenoside Rd(17),notoginsenoside R₁ (18),ginsenoside Re₂ (19), ginsenoside Re(20), 20-gluco-ginsenoside Rf(21),quinquenoside-R₁ (22),ginsenoside Ro methyl ester(23),ginsenoside Ro(24),ginsenoside Rb₁ (25),ginsenoside Rc(26),ginsenoside Rb₂ (27),ginsenoside Ra₂ (28),ginsenoside Ra₃ (29),ginsenoside Rb₃ (30),20(22)Z-ginsenoside Rh₄ (31),chikusetsusaponin IVa butyl ester(32), 20(22)Z-ginsenoside Rs₄ (33),ginsenoside Rs₅ (34),20(22)E-ginsenoside Rs₄ (35),zingibroside R1-6'-butyl ester(36), chikusetsusaponin IVa methyl ester(37),20(S)-ginsenoside Rs₃ (38),20(R)-ginsenoside Rs₃ (39),zingibroside R1-6'-methyl ester(40),ginsenoside Rz₁ (41),ginsenoside Rk₁ (42),ginsenoside Rg₅ (43),23-O-methylginsenoside-Rg₁ ₁ (44),12β,25-dihydroxydammar-20(22)E-ene-3-O-β-D-glucopyranosyl-(1→2)-O-β-D-glucopyranoside(45), 20(22)Z-ginsenoside F₄ (46),3β,12β-dihydroxydammar-20(22)E,24-diene-6-O-β-D-xylopyranosyl-(1→2)-O-β-D-glucopyranoside(47), 20(S)-ginsenoside Rg₃ (48),20(R)-ginsenoside Rg₃ (49),20(22)E-ginsenoside Rg₉ (50),ginsenoside-Ro-6'-butyl ester(51), and polyacetyleneginsenoside Ro(52).
CONCLUSIONS:
Compounds 8, 12, 31-33, 36, 37, 44, 45, 47 and 51 were isolated from the P. ginseng, and compounds 19, 23 and 46 were isolated from Chinese red ginseng for the first time. | | Lishizhen Medicine and Materia Medica Research, 2010. | | Metabolites of Notoginsenoside R_1 in Rats.[Reference: WebLink] | To investigate the metabolites of notoginsenoside R1 in rats.
METHODS AND RESULTS:
The microbial transformation metabolites of notoginsenoside R1 were used as standard references.LC-ESI-MS/MS analysis was used in the metabolism studies of notoginsenoside R1 in rats. The structures of eight metabolites of notoginsenoside R1 in rat feces were identified by comparison with the reference standards separated from biotransformation.Their structures were identified as 20(S)-notoginsenoside R2, 20(R)-Notoginsenoside R2, 20(S)-ginsenoside Rh1,20(R)-ginsenoside Rh1,ginsenoside Rh4,protopanaxatriol,ginsenoside F1 and 3β,12β-dihydroxydammar-E-20(22)-24-diene-6-O-β-D-xylopyranosyl-(1→2)-β-D-glucopyranoside.
CONCLUSIONS:
Notoginsenoside R1 is extensively metabolized in rat and has the same metabolic pathway with microorganisms to some extent. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)