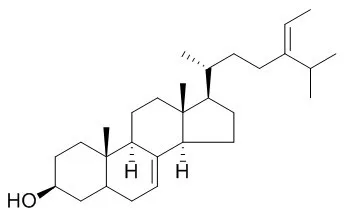
| Structure Identification: |
| Phytochemistry. 2010 Aug;71(11-12):1245-52. | | High throughput screening of mutants of oat that are defective in triterpene synthesis.[Pubmed: 20557911] |
The triterpenes are a large and diverse group of plant natural products that have important functions in plant protection and food quality, and a range of pharmaceutical and other applications. Like sterols, they are synthesised from mevalonate via the isoprenoid pathway, the two pathways diverging after 2,3-oxidosqualene. During triterpene synthesis 2,3-oxidosqualene is cyclised to one of a number of potential products, the most common of these being the pentacyclic triterpene beta-amyrin. Plants often produce complex mixtures of conjugated triterpene glycosides which may be derived from a single triterpene skeleton. The delineation, functional analysis and exploitation of triterpene pathways in plants therefore represent a substantial challenge.
METHODS AND RESULTS:
Here we have carried out high throughput screening to identify mutants of diploid oat (Avena strigosa) that are blocked in the early steps of triterpene synthesis. We also show that mutants that are affected in the first committed step in synthesis of beta-amyrin-derived triterpenes, and so are unable to cyclise 2,3-oxidosqualene to beta-amyrin (sad1 mutants), accumulate elevated levels of primary sterols. The major differences were in Delta-7-campesterol and Delta-7-avenasterol, which both increased several fold relative to wild-type levels.
CONCLUSIONS:
This is presumably due to accumulation of squalene and 2,3-oxidosqualene and consequent feedback into the sterol pathway, and is consistent with previous reports in which specific oxidosqualene cyclase inhibitors and elicitors of triterpene biosynthesis were shown to have inverse effects on the flux through the sterol and triterpene pathways. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)