| In vitro: |
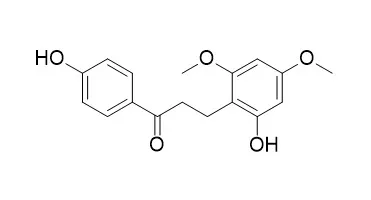
| Planta Med. 1997 Dec;63(6):540-543. | | Retrodihydrochalcones and homoisoflavones isolated from Thai medicinal plant Dracaena loureiri and their estrogen agonist activity[Pubmed: 9434606] | | Biological evaluation of the extract prepared from the stem wood of Dracaena loureiri, a Thai folkloric medicine called "Chan-daeng", revealed a significant capacity to inhibit [3H]-estradiol binding to the estrogen receptor. During the course of separation, two novel (1 and 2) and two known retrodihydrochalcones (3 and 4), in addition to three known homoisoflavones (5, 6, and 7), were isolated. The structures of compounds 1 and 2 were established by NMR and MS studies by correlating their spectroscopic properties with those of 3 and 4. Each isolate was assessed for its estrogenic activity. Compounds 1 and 6 exhibited activity comparable to that of genistein and daidzein. | | Planta Med . 2002 Sep;68(9):841-843. | | Flavonoids and stilbenoids with COX-1 and COX-2 inhibitory activity from Dracaena loureiri[Pubmed: 12357401] | | Fom the stem wood of Dracaena loureiri, a new homoisoflavanone named loureiriol (1) and eight known flavonoid and stilbenoid derivatives, including 5,7-dihydroxy-3-(4-hydroxybenzyl)-4-chromanone (2), 4,4'-dihydroxy-2,6-dimethoxydihydrochalcone (3), 2,4'-dihydroxy-4,6-dimethoxydihydrochalcone (4), 4'-hydroxy-2,4,6-trimethoxydihydrochalcone (5), 4,6,4'-trihydroxy-2-methoxydihydrochalcone (6), 4,3',5'-trihydroxystilbene (7), 4,3'-dihydroxy-5'-methoxystilbene (8) and 4-hydroxy-3',5'-dimethoxystilbene (9) were isolated. These compounds were evaluated for their inhibitory activity against the enzymes cyclooxygenase-1 and cyclooxygenase-2. Potent but non-selective activity was found for the stilbenoids 7-9 (IC(50) 1.29 - 4.92 microM) whereas weak or no activity was observed for the flavonoids 1-6. |
|





 Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019)
Cell. 2018 Jan 11;172(1-2):249-261.e12. doi: 10.1016/j.cell.2017.12.019.IF=36.216(2019) Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019)
Cell Metab. 2020 Mar 3;31(3):534-548.e5. doi: 10.1016/j.cmet.2020.01.002.IF=22.415(2019) Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)
Mol Cell. 2017 Nov 16;68(4):673-685.e6. doi: 10.1016/j.molcel.2017.10.022.IF=14.548(2019)